Allodynia
Top Contributors - Parker Hall, Melissa Coetsee, Abbi Davis, Jessica Bradshaw, Olajumoke Ogunleye and Kim Jackson
Introduction[edit | edit source]
Allodynia is defined by the International Association for the Study of Pain (IASP) as
"Pain resulting from a stimulus that does not normally provoke/elicit pain" [1]
For example, brushing a feather against the arm causes pain where it should only cause a sensation.[2] It is a clinical term to describe this phenomenon of altered quality of a sensation, and does not imply a mechanism or specific diagnosis.[1]It may however give insight into possible mechanisms at play.
Classification[edit | edit source]
Allodynia is categorised into four types[3]:
- Dynamic mechanical when pain results from an object moving across the skin (stroking or brushing); mediated by A-beta fibres
- Thermal which results from mild temperature changes; mediated by A-delta and C-fibres
- Tactile (or static) which results from gentle touch or pressure; mediated by A-delta nociceptive fibres
Aetiology/Mechanism[edit | edit source]
Allodynia is considered a sign/symptom, not a disease. It may be a temporary 'normal' (adaptive) response to tissue damage for better protection of vulnerable tissues.[4] Changes in nervous system processing can result in allodynia persisting long after the original injury has healed, and allodynia can also occur in the absence of injury. The pain associated with allodynia is then regarded as maladaptive and can have negative effects on recovery and quality of life. [5]Although the exact mechanisms underlying allodynia is the subject of ongoing research, the most accepted hypotheses are presented below.
The mechanisms responsible for allodynia include:
- Sensitisation of dorsal horn cells: Increased excitability of second order neurons in the spinal cord[6]
- Sensitisation of mechanoreceptors: Low threshold A-beta fibres become sensitised. These fibres are usually not nociceptive (rather mechanoreceptors). A-beta fibres communicate with and activate nociceptors (A-delta fibres) through various mechanisms (phenotypic switching, sprouting and opening of postsynaptic excitatory pathways), resulting in pain when non-painful mechanical stimuli is applied. As a result there is a loss of segregation of touch and pain. [2][4][7]
- Central inhibition errors: A change in the balance of descending inhibitory and facilitating pathways from the brain to the spinal cord can result in central sensitisation[8]. Mental state can influence the perception of allodynia by altering the inhibition of nociceptive input.
These mechanisms can be facilitated by persistent peripheral nociceptive input, resulting in sensitisation, or can be as a direct result of damage/disease of the peripheral or central nervous system.[6]
Conditions[edit | edit source]
Allodynia is often associated with conditions that involve sensitisation of the skin. Common examples include sunburn, inflammation or trauma.[1]Allodynia is a normal protective response after tissue injury has occurred and will usually subside as healing progresses. It may however increase over time in certain conditions, such as neuropathic pain conditions.[1]Certain types of allodynia (eg. thermal allodynia) are more often associated with specific conditions - for example, cold allodynia is very common in post-stroke pain and chemotherapy neuropathy, whereas mechanical allodynia is more common in neuralgias and other neuropathies .[6]
Listed below are some conditions that may present with allodynia:
- Neuropathic Pain: Allodynia is present in 15-50% of patients with neuropathic pain[2]
- Neuropathies: Eg. Carpal Tunnel Syndrome
- Polyneuropathy such as HIV-related Neuropathy and Diabetic Neuropathy: Often bilateral, distal and symmetrical allodynia
- Brachial Plexus Injury
- Postherpetic Neuralgia
- Fibromyalgia[6]
- Trigeminal Neuralgia and postherpetic neuralgia[6]
- Chemotherapy induced peripheral neuropathy
- Migraine: Cutaneous allodynia is present in about 60% of people suffering from migraines and indicates central sensitisation.[3]
- Central Sensitisation
- Peripheral Sensitisation
- Complex Regional Pain Syndrome (CRPS)
- Chronic Low Back Pain
- Osteoarthritis[6]
- Rheumatoid Arthritis
- Post-amputation stump pain[6]
- Central nervous system disorders: Post-stroke pain, Multiple Sclerosis, Spinal Cord Injury[6]
Differential Diagnosis[edit | edit source]
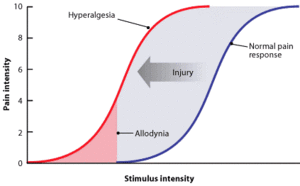
Another clinical term that needs to be differentiated from allodynia, is hyperalgesia. Where allodynia refers to changes in the quality of sensation, hyperalgesia refers to changes in the intensity of the sensation of pain.[1]
Hyperalgesia is the condition of having increased sensitivity to pain or enhanced intensity of pain sensation. There is an exaggerated experience of pain from a stimulus that is normally painful - i.e. an increased response/pain on supra-threshold stimulation (see image)[1] [4].
Although allodynia and hyperalgesia are distinct clinical terms, they can and often do co-exist.[2]
Assessment[edit | edit source]
Subjective Assessment[edit | edit source]
- Obtain a history of diabetes, herpes, chemotherapy, HIV, surgical procedures - any of these could contribute to neuropathic pain
- Take note of current medications as some medications can cause neuropathic pain (eg. antiretroviral treatment and chemotherapy)
- Is the pain associated with touch or stroking or temperature changes?[2]
- Mental health screening to determine stress levels and detect depression and/or anxiety - this could affect central inhibition pathways
Objective Assessment[edit | edit source]
- Always compare to the unaffected side or a body site distant from the affected area (especially if there is bilateral involvement)
- Light touch: With the patient's eyes closed, use a cotton swab/Q-tip to gently stroke the affected area and then the opposite side/unaffected and note whether the patient feels pain
- Temperature: Use a cold metal coin for cold, and then heat it in your pocket for heat - determine whether the patient feels pain with cold/hot touch
- If allodynia is present, determine whether it follows a dermatomal or peripheral nerve pattern, and whether it is bilateral
- Testing for allodynia forms part of Quantitative Sensory Testing - see the page for a more detailed description of assessment and interpretation
- If allodynia is present, a neurological examination (reflexes, muscle strength etc.) is indicated to determine whether nerve damage could be the underlying cause
Treatment[edit | edit source]
Providers shoulder treat/manage the underlying condition that is causing the allodynia, as well as manage the pain associated with it (also see Neuropathic Pain). It is important to remember that various mechanisms can be involved in the central sensitisation that allodynia is a sign of. The presence of allodynia should alert the clinician to investigate nerve pathology and central mechanisms, and treatment should target the mechanisms involved.
- Pharmacologic: Anticonvulsants (such as gabapentin), triptans (used to treat migraines), and some antidepressants are most effective for neuropathic conditions. NSAIDs may also be effective where persistent inflammation is present.[6] Topical creams that can help manage allodynia will typically contain lidocaine or capsaicin as the active ingredient, but there is limited evidence of its effectiveness.[2] Opioids should be avoided.
- Psychosocial interventions: Counselling may be recommended if signs of depression are detected. Biofeedback, mindfulness training, and cognitive behavioural therapy can influence descending inhibition and therefore alter pain perception.
- Physiotherapy: The psychologically informed physiotherapist can include pain neuroscience education (PNE) and cognitive functional therapy. Physiotherapist can help manage allodynia with desensitisation and/or graded motor imagery.
- Other procedures: A nerve block injection may be recommended to reduce pain in a specific nerve or nerve group.[2]
Conclusion[edit | edit source]
Allodynia is a clinical sign that indicates sensitisation of the nervous system. It is common in neuropathic and chronic pain conditions, and may be influenced by emotional state. It can also have a significant negative impact on quality of life and requires early detection and multidisciplinary management.
Resources[edit | edit source]
12 Item Allodynia Symptom checklist (for Migraine)
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 IASP. Terminology. Available from: https://www.iasp-pain.org/resources/terminology/ (accessed 12 Dec 2023)
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 He Y, Kim PY. Allodynia [Internet]. PubMed. Treasure Island (FL): StatPearls Publishing; 2016 [cited 2022 Apr 9]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537129/
- ↑ 3.0 3.1 Mínguez-Olaondo A, Quintas S, Morollón Sánchez-Mateos N, López-Bravo A, Vila-Pueyo M, Grozeva V, Belvís R, Santos-Lasaosa S, Irimia P. Cutaneous allodynia in migraine: a narrative review. Frontiers in neurology. 2022 Jan 21;12:831035.
- ↑ 4.0 4.1 4.2 Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiological reviews. 2009 Apr;89(2):707-58.
- ↑ Viana F. Nociceptors: thermal allodynia and thermal pain. InHandbook of clinical neurology 2018 Jan 1 (Vol. 156, pp. 103-119). Elsevier.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. The Lancet Neurology. 2014 Sep 1;13(9):924-35.
- ↑ Kuner R, Flor H. Structural plasticity and reorganization in chronic pain. Nature Reviews Neuroscience. 2017 Jan;18(1):20-30.
- ↑ Kuner R. Central mechanisms of pathological pain. Nature medicine. 2010 Nov;16(11):1258-66.