Quantitative Sensory Testing (QST)
Original Editor - Melissa Coetsee
Top Contributors - Melissa Coetsee and Carina Therese Magtibay
Introduction[edit | edit source]
Quantitative sensory testing (QST) is a systematic psychophysical test method used to measure sensory thresholds for pain, touch, vibration, and temperature sensations.[1]It quantifies individual sensory perceptions using direct patient feedback. It tests for sensory loss (hypoesthesia, hypoalgesia) and sensory gain (hyperesthesia, hyperalgesia, allodynia)[2] and thus tests nociceptive and non-nociceptive properties of different afferent nerve fibres and central pathways[3].
Clinical Application[edit | edit source]
QST can be used to evaluate any condition that affects sensory function - it may help with diagnosis and disease monitoring. QST can be very useful to identify underlying contributing pain mechanisms and pathophysiology, which can assist with targeted intervention strategies[2][3]. It is best to compare results with normative values, and it is best conducted early after onset of a condition - the reason for this is that people with central sensitisation will present with widespread QST abnormalities, rendering the contralateral side an inaccurate comparison site. In early onset cases, the contralateral side is more likely to still reflect the patient's norm, but if predisposing existing sensory abnormalities are present it could interfere with interpretation.
Conditions for which QST can be useful include[2][3]:
- Neuropathic pain
- Polyneuropathy (diabetic, HIV-related, chemotherapy-related)
- Postherpetic neuralgia
- Complex regional pain syndrome (CRPS)
- Chronic low back pain
- Knee osteoarthritis
Components[edit | edit source]
To perform QST, a patient is stimulated with quantified sensory stimuli and based on patient feedback, perception thresholds are identified for the following sensory functions: light touch, pressure, vibration, thermal sensations, heat and cold pain. This way A-delta fibres (small diameter myelinated) and C-fibres (unmyelinated) are assessed. The categories of the full test include[4]:
- Thermal detection thresholds for the perception of cold, warm and paradoxical heat sensations
- Thermal pain thresholds for cold and hot stimuli
- Mechanical detection thresholds for touch and vibration
- Mechanical pain sensitivity including:
- Pin-prick thresholds
- Blunt pressure thresholds
- Stimulus response for pin-prick sensitivity and mechanical allodynia
- Pain summation to repetitive pin prick stimuli
Procedure[edit | edit source]
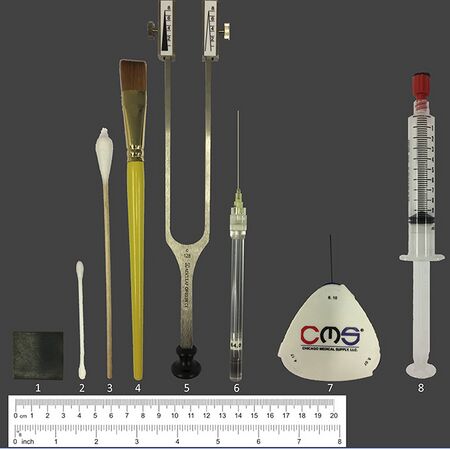
Sophisticated computerised equipment is often used in clinical trials, but hand-held tools are also available for clinical use[1]. The following table presents the standardised procedure as recommended by the German Research Network on Neuropathic Pain. For fully detailed description of this procedure see the Resources section. Simplified versions will be discussed later:
| Test | Equipment | Nerves tested | Instructions | Measurement |
|---|---|---|---|---|
| Thermal Testing | Thermode with element and cooling water system | A-delta fibres and C-fibres | Thermode is placed on the skin and measure heat and pain & cold and pain detection between 0 and 50 degrees Celsius | Average of three consecutive measures |
| Mechanical Detection | Von Frey Filaments | A-beta fibres | Contact with skin for 2 seconds determine at what point the patient detects tactile input | Average of five above and below-threshold stimulus intensities |
| Mechanical pain threshold | Needle stimulators | A-delta fibre subtypes | Needle applied perpendicular to the skin in ascending and descending stimulus intensity with skin contact time of 1-2s | Average of five above and below-threshold stimulus intensities |
| Mechanical pain sensitivity | Needle stimulators, Q-tip, soft brush, cotton pad | Allodynia and pin-prick hyperalgesia (central pathways) | Gentle stimuli applied to the 2cm of skin (stroking) with the various instruments | Subjects are asked to rate the perception of the stimulus using a pain scale of 0-100 |
| Vibration detection threshold | Tuning fork | A-beta fibre | Place fork over bony prominence until subject can not feel vibration any more | Disappearance threshold taken on 3 repetitions |
| Pressure pain threshold | Pressure gauge device (Wagner instruments) | Deep somatosensory function | Apply gradually increasing pressure over muscle area | 3 series of ascending stimulus intensities |
| Temporal Summation | Needle stimulator (pin prick) | Wind-up phenomenon (spinal cord neurons) | A series of 10 repetitive pinprick stimuli of the same intensity | Subjects are asked to rate the perception of the stimulus using a pain scale of 0-100 |
Normal values[edit | edit source]
Reference values have been established for some ethnic groups and body parts and can be found in journal articles[5][4]. These reference ranges are however based on very accurate computerised measuring tools and hard to use in clinical practice. Factors that affect normal values[3]:
- Gender: men and women differ in their sensitivity to pain; women are significantly more sensitive to pain with QST testing
- Age: aging is correlated with an increase in perception and pain threshold
- Body site: sensitivity will vary according to body sites
Bed-side testing[edit | edit source]
The full protocol of QST is not practical for regular use in clinical practice, as it is time-consuming and requires expensive equipment. Simplified modifications of these tests may however provide useful insights, despite reduced accuracy. The reliability and validity of bedside QST tests have been demonstrated for various conditions[6][7][8]. The following affordable and quick bedside methods have been suggested and may be valuable to gain understanding regarding a patient's underlying pain mechanisms[6]:
| Test | Equipment | Instructions |
|---|---|---|
| Thermal tests | Metal iron piece (square of 3cm); Cold detection: Metal piece at room temp (about 22C); Cold pain: Cool metal piece in a fridge (8C); Warm detection: Warm metal using a milk heater to37C; Heat pain: Warm to 45C | Apply metal piece to skin for 3s; Subjects rate the stimulus as painful or not, and rate pain from 0-10; They also report whether the stimulus was felt as hot or cold and rate the intensity from 0-10 |
| Alternative thermal tests[7] | Detection:Use metal coins at room temp/ heated in pocket of trousers | Pain: ice cubes in plastic bag; glass vial filled with hot tap water (place for 10sec) |
| Mechanical detection (dynamic) | Q-tip (cotton swab)
Von Frey filaments can used for static detection[9] |
Q-tip: Stroke skin area; subjects indicate the intensity from 0-20 (10 means equal to control area, <10 feels less intense) in comparison with a control area |
| Mechanical pain sensitivity | Pin-prick using CMS/Von Frey hair/Neuropen with neurotip[8]; toothpick/ paperclip [9] | Apply light prick to affected skin and control area; subjects report whether it felt sharp or blunt, and rate pain from 0-10 |
| Temporal summation | Pin-prick using CMS/Von Frey hair; toothpick can also work | 10 repeated pin pricks with a frequency of 1/sec; subject rates pain intensity of first and last prick from 0-10 |
| Mechanical allodynia | Brush, cotton swab or cotton wisp | Draw a cross with 2 strokes, 4x; subjects report on whether the stimulus is painful and rate between 0-10 |
| Pressure pain sensitivity | 10ml syringe sealed with a plug and felt; or algometer
Alt: Pencil with eraser/thumb[9] |
Apply gradual pressure to muscle, up to 4ml; subjects report on whether the stimulus is painful and rate between 0-10 |
| Vibration detection | Tuning fork (128Hz) | Place fork over bony prominence in the affected and a control area; subjects indicate when the stimulus vanished |
Complementary Tests[edit | edit source]
- Temporal summation (TS): Apply rapidly repeated mechanical stimuli (for 5-10s) to a painful region - if TS is present it will result in an increased pain perception indicating central sensitisation[2]. Although it forms part of the QST protocol it can be used in isolation to detect increased firing of secondary spinal cord neurons. Pressure or pin-prick can be used as a stimulus.
- 2-point discrimination: Especially useful in the upper limb to test tactile perception. Can be impaired in both PNS and CNS conditions. In the absence of peripheral pathology, it could indicate cortical changes in the homunculus.
- Conditional pain modulation test (CPM): CPM tests the function of central pain inhibition/facilitation, and is based on the phenomenon that "pain inhibits pain". It involves applying an initial noxious stimulus (thermal or mechanical), which is measured before and after applying a second stimulus (cold or hot water bath applied to a distal extremity) - if descending inhibitory control is functioning properly, the initial source of pain will be less intense during the application of the remote stimulus. [2]
Interpretation[edit | edit source]
Overall, QST tests for[7]:
- Loss of sensory function - indicative of demyelination or axon degeneration
- Heat, cold, mechanical detection and/or vibration thresholds have reduced sensation
- Gain of sensory function - indicative of neuronal hyperexcitability, such as lack of inhibition
- Cold/heat, mechanical pain and/or pressure pain thresholds have increased sensation
- Wind up and allodynia is present
The table below summarises the link between underlying pain mechanisms / pathophysiology and specific QST findings:
| Underlying mechanism/ Sign | QST finding |
|---|---|
| Hypoesthesia (sensory loss) | Elevated sensory thresholds, i.e. decreased sensitivity to non-noxious stimuli |
| Hypoalgesia | Elevated sensory thresholds, i.e. decreased sensitivity to noxious stimuli |
| Hyperalgesia | Increased sensitivity (lowered thresholds) to heat, cold, pinprick or deep pressure (noxious stimuli) |
| Allodynia | Pain in response to non-noxious stimuli on skin |
| Peripheral sensitisation | Heat hyperalgesia (lowered hot pain threshold)
Lowered pain thresholds for pressure/pin prick proximal to painful area or along nerve distribution / segmental region |
| Central sensitisation | Increased sensitivity to cold/pin prick/pressure/cold hyperalgesia that is widespread and contralateral; Mechanical allodynia; Temporal summation (repeated mechanical and thermal stimuli causes increased pain) |
| Polyneuropathy | All thresholds elevated |
| Small fibre neuropathy | Vibration thresholds are normal, but others elevated |
| Nerve deafferation | Reduced sensitivity with all tests |
The table below summarises QST findings for specific conditions, as identified in research studies[2]. It is however important to remember that different pain mechanisms may operate in patients with the same disease, and the same pain mechanisms can be present in patients with different diseases[9]:
| Condition | QST findings | Implication |
|---|---|---|
| Chronic low back pain | (1) Increased sensitivity (decreased threshold) to heat and pressure
(2) Temporal summation |
(1) Indicates peripheral sensitiasation
(2) Central sensitisation components present |
| Lower limb fractures | Decreased sensitivity (elevated threshold) to touch and increased sensitivity (decreased threshold) to warmth | Identifies underlying peripheral nerve injury which can affect limb function and balance |
| Knee OA | (1) Increased sensitivity (lowered thresholds) to pressure around the knee
(2) Increased sensitivity to pressure and cold at distal sites; Temporal summation (TS with pinprick |
(1) Indicates peripheral sensitisation
(2) Indicates central sensitisation - predicts poor surgery outcomes if not addressed; will likely not respond fully to NSAIDs |
| Neuropathic pain[6] | Hypersensitivity to heat; loss of tactile perception; allodynia and wind-up present | Combination of sensory loss signs and hyperalgesia are strong indicators of potential neuropathic pain mechanisms |
| Whiplash associated disorder[7] | Cold hyperalgesia
Increased pain sensitivity |
If these QST findings ae present it may predict poor recovery |
Limitations[edit | edit source]
- QST abnormalities are present in non neuropathic pain, making it difficult to use QST as a definitive tool for identifying neuropathic pain[1]
- Abnormal findings are not specific for peripheral nerve dysfunction, as central nervous system disorders will also alter sensory thresholds[1].
- It is a subjective psychophysical test, entirely dependent on patient alertness, motivation and willingness to supply accurate feedback. There is large intra- and interindividual variation[1]
Summary[edit | edit source]
QST can be a very valuable clinical tool in patients with chronic pain by assisting to characterise pain experiences and underlying mechanisms. It provides information about potential underlying mechanisms contributing to pain, therefore incorporating QST may improve the ability to implement individualised treatment plans based on underlying mechanisms[2].
Resources[edit | edit source]
- Quantitative Sensory Testing - standardised full procedure
- QST for low back pain - bedside QST combined with other examination methods
- QST for neuropathic pain - supplemental material with description of bedside techniques
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Horowitz SH. Neuropathic pain: is the emperor wearing clothes. Current Therapy in Pain, WB Saunders. 2009:9-14.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Weaver KR, Griffioen MA, Klinedinst NJ, Galik E, Duarte AC, Colloca L, Resnick B, Dorsey SG, Renn CL. Quantitative sensory testing across chronic pain conditions and use in special populations. Frontiers in Pain Research. 2022 Jan 28;2:779068.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Mucke M, Cuhls H, Radbruch L, Baron R, Maier C, Tolle T, Treede RD, Rolke R. Quantitative sensory testing (QST). English version. Schmerz. 2016;35:153-60.
- ↑ 4.0 4.1 Rolke R, Baron R, Maier CA, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Bötefür IC, Braune S. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain®. 2006 Aug 1;123(3):231-43.
- ↑ González‐Duarte A, Lem‐Carrillo M, Guerrero‐Torres L. Normative values of quantitative sensory testing in Hispanic Latino population. Brain and Behavior. 2016 Jul;6(7):e00466.
- ↑ 6.0 6.1 6.2 6.3 Reimer M, Forstenpointner J, Hartmann A, Otto JC, Vollert J, Gierthmühlen J, Klein T, Hüllemann P, Baron R. Sensory bedside testing: a simple stratification approach for sensory phenotyping. Pain Reports. 2020 May;5(3).
- ↑ 7.0 7.1 7.2 7.3 Zhu GC, Böttger K, Slater H, Cook C, Farrell SF, Hailey L, Tampin B, Schmid AB. Concurrent validity of a low‐cost and time‐efficient clinical sensory test battery to evaluate somatosensory dysfunction. European Journal of Pain. 2019 Nov;23(10):1826-38.
- ↑ 8.0 8.1 Sachau J, Appel C, Reimer M, Sendel M, Vollert J, Hüllemann P, Baron R. Test–retest reliability of a simple bedside-quantitative sensory testing battery for chronic neuropathic pain. Pain Reports. 2023 Jan;8(1).
- ↑ 9.0 9.1 9.2 9.3 Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, Scoffings D, Phillips A, Guo J, Laing RJ, Abdi S. A novel tool for the assessment of pain: validation in low back pain. PLoS medicine. 2009 Apr 7;6(4):e1000047.