Heterotopic Ossification: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
'''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}</div> | '''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}</div> | ||
== Introduction == | == Introduction == | ||
Heterotopic ossification is frequently observed in the [[Rehabilitation Contexts|rehabilitation]] population. It consists of the formation of mature, lamellar [[bone]] in extraskeletal soft tissue where bone does not usually exist. Patient populations at risk of developing heterotopic ossification include patients with [[Burns Overview|burns]], [[Stroke|strokes]], [[Spinal Cord Injury|spinal cord injuries]], traumatic [[amputations]], joint replacements, and [[Traumatic Brain Injury|traumatic brain injuries]].<ref>Sun E, Hanyu-Deutmeyer AA. [https://www. | Heterotopic ossification is frequently observed in the [[Rehabilitation Contexts|rehabilitation]] population. It consists of the formation of mature, lamellar [[bone]] in extraskeletal soft tissue where bone does not usually exist. Patient populations at risk of developing heterotopic ossification include patients with [[Burns Overview|burns]], [[Stroke|strokes]], [[Spinal Cord Injury|spinal cord injuries]], traumatic [[amputations]], joint replacements, and [[Traumatic Brain Injury|traumatic brain injuries]].<ref name=":0">Sun E, Hanyu-Deutmeyer AA. [https://www.ncbi.nlm.nih.gov/books/NBK519029/ Heterotopic Ossification].Available: https://www.ncbi.nlm.nih.gov/books/NBK519029/<nowiki/>(accessed 24.10.2021)</ref> | ||
* Lesions range from small clinically insignificant foci of ossification to large deposits of bone that cause pain and restriction of function. | |||
* The most common presentation is with pain around the ossification site<ref>Radiopedia [https://radiopaedia.org/articles/heterotopic-ossification HO] Availasble: https://radiopaedia.org/articles/heterotopic-ossification<nowiki/>(accessed 24.10.2021)</ref> | |||
<br> | <br> | ||
| Line 17: | Line 20: | ||
<br> | <br> | ||
== Etiology | == Etiology == | ||
The exact mechanism of HO in traumatic and neurogenic HO is unknown, but 2 common factors precede the formation of HO, the first being trauma or an inciting neurological event. | |||
# Traumatic (fracture, arthroplasty, muscular trauma, joint dislocation, burns). In total joint arthroplasty, HO most commonly occurs for the [[Total Hip Replacement|replacement of hips]], [[Total Knee Arthroplasty|knee]]<nowiki/>s, elbow, and [[Total Shoulder Arthroplasty|shoulder]]. Chronic muscular trauma leads to what is traditionally known specifically as traumatic [[Myositis Ossificans of the Quadriceps|myositis ossificans]]. The most common sites for traumatic myositis ossificans are the [[Quadriceps Muscle|quadriceps femoris]] muscle and the [[brachialis]] muscle. | |||
# Neurogenic (stroke, SCI, TBI, brain tumors). The most common sites for neurogenic HO are the hips, elbows (extensor side), shoulders, and knees. Uncommon sites of HO that may be encountered in a rehabilitation setting are incisions, [[Kidney|kidneys]], uterus, corpora cavernosum, and the gastrointestinal (GI) tract. The exact cause and mechanism of neurogenic HO are unknown. | |||
Risk factors for developing HO | |||
* [[Spasticity]], [[Older People - An Introduction|older age]], [[Pressure Ulcers|pressure ulcer]], the presence of [[Deep Vein Thrombosis|deep vein thrombosis]] (DVT), having a tracheostomy, long bone [[Fracture|fractures]], prior injury to the same area, [[Edema Assessment|edema]], immobility, long-term [[Coma Recovery Scale (Revised)|coma]], and severity of injury (trauma, TBI, SCI, stroke). | |||
* High/moderate risk factors in the THA population include men with bilateral THA, prior history of HO, [[Ankylosing Spondylitis (Axial Spondyloarthritis)|ankylosing spondylitis]], diffuse idiopathic hyperostosis, or [[Paget's Disease|Paget's]] disease.<ref name=":0" /> | |||
== Epidemiolgy == | |||
* HO is twice as common in males versus females, but it is noted that females older than 65 years old have an increased risk of developing HO. | |||
* The incidence of neurogenic HO is 10% to 20%.<ref name=":0" /> | |||
* Following lower extremity amputation: 7%<sup><ref name="p6">Hsu JE, Keenan MA. Current review of heterotopic ossification. UPOJ 2010; 20: 126-130.</ref></sup> | |||
* Following SCI: 20% (ranges reported from 20-40%)<sup><ref name="p6" /></sup> | |||
* Following THA: 55%<sup><ref name="p1">Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic Ossification Revisited. Orthopedics. 2011Jan;34(3):177.</ref></sup> | |||
* Following elbow fracture and/or dislocation: 90%<ref name="p8">Dalury DF, Jiranek WA. The incidence of heterotopic ossification after total knee arthroplasty. Journal of Arthroplasty 2004; 19: 447-457.</ref><br> | |||
== Clinical Diagnosis == | |||
== | Clinical signs and symptoms of HO may appear as soon as 3 weeks or up to 12 weeks after initial musculoskeletal trauma, spinal cord injury, or other precipitating events.<sup><ref name="p9">Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. Jour of Nuclear Medicine 2002; 43: 346-353.</ref></sup> The first sign of HO is generally loss of joint mobility and subsequently loss of function. Other findings that may suggest the presence of HO include swelling, erythema, heat, local pain, palpable mass, and contracture formation. In some cases, a fever may be present.<sup><ref name="p1" /> </sup> | ||
Presentation | |||
# Symptoms: painless loss of ROM, interferes with ADL, CRPS symptoms, fever | |||
# Inspection: warm, painful, swollen joint; may have effusion; skin problems (decubitus ulcers from contractures around skin, muscles, ligaments, skin maceration and hygiene problems) | |||
# Motion: decreased joint ROM; joint ankylosis; with HO after TKA, might develop quad muscle snapping or patella instability | |||
# Neurovascular: peripheral neuropathy; HO often impinges on adjacent NV structures<ref>Orthobullets [https://www.orthobullets.com/pathology/8044/heterotopic-ossification HO] Available: https://www.orthobullets.com/pathology/8044/heterotopic-ossification<nowiki/>(accessed 24.10.2021)</ref> | |||
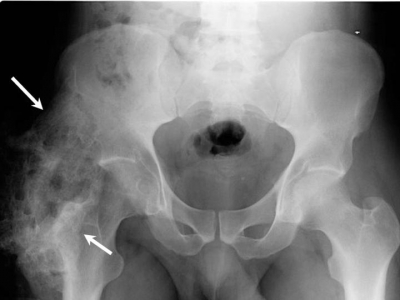
[[Image:Heterotopic.jpg|frame|right|Heterotopic ossification progression of the hip]] | |||
== '''Differential Diagnoses''' == | |||
The initial inflammatory phase of HO may mimic other pathologies such as cellulitis, thrombophlebitis, osteomyelitis, or a tumorous process.<sup><ref name="p3" /></sup><ref name="p4">Bossche LV, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005; 37: 129-136.5. Pape HC et al. Current concepts in the development of hetetrotopic ossification. Journ Bone and Joint Surg 2004; 86: 783-787.</ref> | |||
Other differential diagnoses include DVT, septic arthritis, hematoma, or fracture. DVT and HO have been positively associated. This is thought to be due to the mass effect and local inflammation of the HO, encouraging thrombus formation. The thrombus formation is caused by venous compression and phlebitis.<sup><ref name="p1" /></sup> | |||
<br> | |||
<br> | |||
<br> | |||
== Diagnostic Tests and Lab Tests == | == Diagnostic Tests and Lab Tests == | ||
| Line 103: | Line 86: | ||
== Associated Co-Morbidities <br> == | == Associated Co-Morbidities <br> == | ||
The most common conditions found in conjunction with heterotopic ossification:<sup><ref name="p4" /> <ref name="p2" /></sup> | The most common conditions found in conjunction with heterotopic ossification:<sup><ref name="p4" /> <ref name="p2">McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol 2005; 34: 609-619.</ref></sup> | ||
*Ankylosing spondylitis | *Ankylosing spondylitis | ||
*Rhuematoid arthritis<sup><ref name="p7" /></sup> | *Rhuematoid arthritis<sup><ref name="p7">Foruria AM, Augustin S, Morrey BF, Sanchez-Sotelo Joaquin. Heterotopic Ossification After Surgery for Fractures and Fractures-Dislocations Involving the Proximal Aspect of the Radius or Ulna. The Journal of Bone and Joint Surgery, Incorporated. 2015May15;95-A(10):e66(1)-e66(7).</ref></sup> | ||
*Hypertrophic osteoarthritis | *Hypertrophic osteoarthritis | ||
*Diffuse idiopathic skeletal hyperostosis | *Diffuse idiopathic skeletal hyperostosis | ||
| Line 112: | Line 95: | ||
*Quadriplegia and paraplegia <br> | *Quadriplegia and paraplegia <br> | ||
== | == Treatment == | ||
Current treatment recommendations consist of mobilization with ROM exercises, indomethacin, etidronate, and surgical resection. | |||
Early treatment with a passive range of motion exercises should be implemented once the presence of HO is confirmed to prevent ankylosing of joints. | |||
Absolute treatment consists of surgical resection of mature bone once the HO has fully matured. This can be 12 to 18 months after the initial presentation. | |||
Surgical consultation with an orthopedic surgeon is warranted only if there will be an improvement in function as demonstrated by mobility, transfers, hygiene, and ADLs. | |||
Indomethacin and etidronate are also used to help arrest bone formation in HO, but efficacy in the traumatic brain injury population has not been clearly proven (see below). | |||
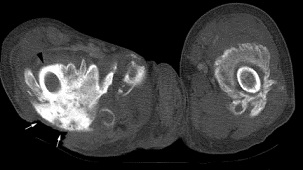
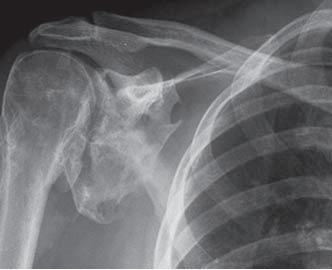
The most effective treatment option in the TBI population is surgical resection. In the SCI population, the most effective NSAID treatment regiments are either Rofecoxib 25 mg per day for 4 weeks or indomethacin 75 mg daily for 3 weeks.<ref name=":0" />[[Image:Shoulder HO CT 2.JPG|frame|right|CT Scan showing heterotopic ossification of the shoulder (Chao et al.)]] | |||
Medications: | |||
The two types of medications shown to have both prophylactic and treatment benefits are as follows: | |||
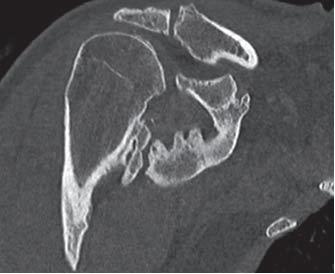
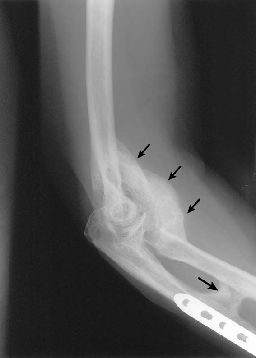
'''Non-steroidal anti-inflammatory drugs: NSAIDS'''<br>Indomethacin (two-fold action)<br>1. Inhibition of the differentiation of mesenchymal cells into osteogenic cells (direct)<br>2. Inhibition of post-traumatic bone remodeling by suppression of prostaglandin-mediated response (indirect) and anti-inflammatory properties[[Image:Shoulder HO Xray 2.JPG|frame|right|AP x-ray of same shoulder as above (Chao et al.)]] | '''Non-steroidal anti-inflammatory drugs: NSAIDS'''<br>Indomethacin (two-fold action)<br>1. Inhibition of the differentiation of mesenchymal cells into osteogenic cells (direct)<br>2. Inhibition of post-traumatic bone remodeling by suppression of prostaglandin-mediated response (indirect) and anti-inflammatory properties[[Image:Shoulder HO Xray 2.JPG|frame|right|AP x-ray of same shoulder as above (Chao et al.)]] | ||
'''Biphosphonates''':<br>Three-fold action<br>1. Inhibition of calcium phosphate precipitation<br>2. Slowing of hydroxyapatite crystal aggregation<br>3. Inhibition of the transformation of calcium phosphate to hydroxyapatite<br> <br> | '''Biphosphonates''':<br>Three-fold action<br>1. Inhibition of calcium phosphate precipitation<br>2. Slowing of hydroxyapatite crystal aggregation<br>3. Inhibition of the transformation of calcium phosphate to hydroxyapatite<br> <br> | ||
<br> | <br> | ||
Sugical intervention | |||
The two main goals of surgical intervention are to alter the position of the affected joint or improve its range of motion (ROM).<ref name="p5" /> {{#ev:youtube|AGewib-kl1A}} | |||
{{#ev:youtube|AGewib-kl1A}} | |||
'''Rehabilitation Post-Operatively''' | '''Rehabilitation Post-Operatively''' | ||
| Line 215: | Line 170: | ||
'''''<u>Clinical Note</u>'': '''The two studies used above were primarily focused on rehabiliatation of the elbow secondary to heterotopic ossification. However, the goals and stages of the rehabilitation process can be used as a guide when treating at other sites. | '''''<u>Clinical Note</u>'': '''The two studies used above were primarily focused on rehabiliatation of the elbow secondary to heterotopic ossification. However, the goals and stages of the rehabilitation process can be used as a guide when treating at other sites. | ||
== Case Report == | |||
== Case Report | |||
'''Title:''' Heterotopic Ossification Causing Radiculopathy after Lumbar Total Disc Arthroplasty | '''Title:''' Heterotopic Ossification Causing Radiculopathy after Lumbar Total Disc Arthroplasty | ||
Revision as of 02:17, 24 October 2021
Original Editors - Bruce Tan as part of the from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Bruce Tan, Morgan Yoder, Hannah McCabe, Naomi O'Reilly, Lucinda hampton, Admin, Elaine Lonnemann, 127.0.0.1, Wendy Walker, WikiSysop and Kim JacksonIntroduction[edit | edit source]
Heterotopic ossification is frequently observed in the rehabilitation population. It consists of the formation of mature, lamellar bone in extraskeletal soft tissue where bone does not usually exist. Patient populations at risk of developing heterotopic ossification include patients with burns, strokes, spinal cord injuries, traumatic amputations, joint replacements, and traumatic brain injuries.[1]
- Lesions range from small clinically insignificant foci of ossification to large deposits of bone that cause pain and restriction of function.
- The most common presentation is with pain around the ossification site[2]
Etiology[edit | edit source]
The exact mechanism of HO in traumatic and neurogenic HO is unknown, but 2 common factors precede the formation of HO, the first being trauma or an inciting neurological event.
- Traumatic (fracture, arthroplasty, muscular trauma, joint dislocation, burns). In total joint arthroplasty, HO most commonly occurs for the replacement of hips, knees, elbow, and shoulder. Chronic muscular trauma leads to what is traditionally known specifically as traumatic myositis ossificans. The most common sites for traumatic myositis ossificans are the quadriceps femoris muscle and the brachialis muscle.
- Neurogenic (stroke, SCI, TBI, brain tumors). The most common sites for neurogenic HO are the hips, elbows (extensor side), shoulders, and knees. Uncommon sites of HO that may be encountered in a rehabilitation setting are incisions, kidneys, uterus, corpora cavernosum, and the gastrointestinal (GI) tract. The exact cause and mechanism of neurogenic HO are unknown.
Risk factors for developing HO
- Spasticity, older age, pressure ulcer, the presence of deep vein thrombosis (DVT), having a tracheostomy, long bone fractures, prior injury to the same area, edema, immobility, long-term coma, and severity of injury (trauma, TBI, SCI, stroke).
- High/moderate risk factors in the THA population include men with bilateral THA, prior history of HO, ankylosing spondylitis, diffuse idiopathic hyperostosis, or Paget's disease.[1]
Epidemiolgy[edit | edit source]
- HO is twice as common in males versus females, but it is noted that females older than 65 years old have an increased risk of developing HO.
- The incidence of neurogenic HO is 10% to 20%.[1]
- Following lower extremity amputation: 7%[3]
- Following SCI: 20% (ranges reported from 20-40%)[3]
- Following THA: 55%[4]
- Following elbow fracture and/or dislocation: 90%[5]
Clinical Diagnosis[edit | edit source]
Clinical signs and symptoms of HO may appear as soon as 3 weeks or up to 12 weeks after initial musculoskeletal trauma, spinal cord injury, or other precipitating events.[6] The first sign of HO is generally loss of joint mobility and subsequently loss of function. Other findings that may suggest the presence of HO include swelling, erythema, heat, local pain, palpable mass, and contracture formation. In some cases, a fever may be present.[4]
Presentation
- Symptoms: painless loss of ROM, interferes with ADL, CRPS symptoms, fever
- Inspection: warm, painful, swollen joint; may have effusion; skin problems (decubitus ulcers from contractures around skin, muscles, ligaments, skin maceration and hygiene problems)
- Motion: decreased joint ROM; joint ankylosis; with HO after TKA, might develop quad muscle snapping or patella instability
- Neurovascular: peripheral neuropathy; HO often impinges on adjacent NV structures[7]
Differential Diagnoses[edit | edit source]
The initial inflammatory phase of HO may mimic other pathologies such as cellulitis, thrombophlebitis, osteomyelitis, or a tumorous process.[8][9]
Other differential diagnoses include DVT, septic arthritis, hematoma, or fracture. DVT and HO have been positively associated. This is thought to be due to the mass effect and local inflammation of the HO, encouraging thrombus formation. The thrombus formation is caused by venous compression and phlebitis.[4]
Diagnostic Tests and Lab Tests[edit | edit source]
Ultrasonography[8]
• Detection of HO 2 weeks earlier than by x-ray
• More accurate than any laboratory tests
• Help clinicians advocate for prompt/aggressive physical therapy
• Eliminates high false-positive rate of physical exam alone
Three-phase bone scintigraphy[9]
• Diagnostic and therapeutic follow-up purposes
• Phases 1 and 2 are indicative of hyperemia and blood pooling (precursors to process of HO)
• Usually positive after 2-4 weeks
• Serial bone scans used to monitor metabolic activity of HO to determine optimal timing for surgical resection and to predict postoperative occurrence
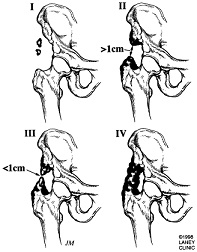
Radiography[4]
• Soft tissue mass is the earliest radiographic finding
• HO seen on radiographs 4-6 weeks post-injury has a typical appearance of circumferential ossification with a lucent center
• Cannot detect the mineralization of HO during first weeks after trauma/onset
• Frequently used to classify HO following THA
• Differential diagnosis: avulsion fracture fragments, osteochondral bodies, nonosseous soft tissue calcification and osteosarcoma
MRI and CT Scan[4]
• MRI not routinely used for evaluation of HO
• CT may detect soft tissue ossification at earlier stages than standard radiograph
Three-phase technetium-99m (99mTc) methylene diphosphonate bone scan[4]
• Most sensitive imaging modality for early detection and assessing the maturity of HO
• Can use to monitor the metabolic activity of HO and determine appropriate time for surgery and predict postoperative recurrence
• Usually positive >2 weeks before radiographic evidence of HO
Prostaglandin E2: (PGE2)[9]
• Monitior PGE2 excretion in 24-hour urinalysis
• PGE2 felt to be reliable bone marker for early detection and determining treatment efficacy
• A sudden increase is an indication for bone scintigraphy
Alkaline Phosphatase: (ALP)[10]
• Frequently used in early detection of HO
ALP values are increased in early HO and plateau at ~4 weeks[8]
• Cannot be used to draw clinical conclusions about maturity or recurrence of HO
• Acute fractures often have similar ALP values, limiting the usefulness of ALP values in diagnosis of HO[8]
Elevated Creatine Kinase Levels[4]
• Correlate with histologic involvement of muscle and severity of disease
• Not specific to HO
• May predict a higher risk for HO development and severity
• May be helpful in planning and evaluation of response to treatment
Matrix Metalloproteinase-9[8]
• Marine animal models suggest this could be an early biomarker for HO formation
Clinical Note: the lack of simple objective measures in detecting heterotopic bone formation causes HO to be misdiagnosed in the early stages, leading to delayed treatment.[3]
Systemic Involvement[edit | edit source]
Evidence shows that there are no systemic effects secondary to the formation of heterotopic ossification. The most common sites where this condition presents are the hips, knees, spine, elbow, wrists, hands, and any site which is involved in a traumatic event.
Associated Co-Morbidities
[edit | edit source]
The most common conditions found in conjunction with heterotopic ossification:[9] [11]
- Ankylosing spondylitis
- Rhuematoid arthritis[12]
- Hypertrophic osteoarthritis
- Diffuse idiopathic skeletal hyperostosis
- Paget's disease
- Quadriplegia and paraplegia
Treatment[edit | edit source]
Current treatment recommendations consist of mobilization with ROM exercises, indomethacin, etidronate, and surgical resection.
Early treatment with a passive range of motion exercises should be implemented once the presence of HO is confirmed to prevent ankylosing of joints.
Absolute treatment consists of surgical resection of mature bone once the HO has fully matured. This can be 12 to 18 months after the initial presentation.
Surgical consultation with an orthopedic surgeon is warranted only if there will be an improvement in function as demonstrated by mobility, transfers, hygiene, and ADLs.
Indomethacin and etidronate are also used to help arrest bone formation in HO, but efficacy in the traumatic brain injury population has not been clearly proven (see below).
The most effective treatment option in the TBI population is surgical resection. In the SCI population, the most effective NSAID treatment regiments are either Rofecoxib 25 mg per day for 4 weeks or indomethacin 75 mg daily for 3 weeks.[1]
Medications:
The two types of medications shown to have both prophylactic and treatment benefits are as follows:
Non-steroidal anti-inflammatory drugs: NSAIDS
Indomethacin (two-fold action)
1. Inhibition of the differentiation of mesenchymal cells into osteogenic cells (direct)
2. Inhibition of post-traumatic bone remodeling by suppression of prostaglandin-mediated response (indirect) and anti-inflammatory properties
Biphosphonates:
Three-fold action
1. Inhibition of calcium phosphate precipitation
2. Slowing of hydroxyapatite crystal aggregation
3. Inhibition of the transformation of calcium phosphate to hydroxyapatite
Sugical intervention
The two main goals of surgical intervention are to alter the position of the affected joint or improve its range of motion (ROM).[10]
Rehabilitation Post-Operatively
It is recommended that a rehabilitation program should start within the first 24 hours after surgery. The program should last for 3 weeks to prevent adhesion.[12]
Physical Therapy Management
[edit | edit source]
Physical therapy has been shown to benefit patients suffering from heterotopic ossification. Pre-operative PT can be used to help preseve the structures around the lesion. ROM exercises (PROM, AAROM, AROM) and strengthening will help prevent muscle atrophy and preserve joint motion.
Clinical note: caution must be taken when working with patients with known heterotopic lesions. Therapy which is too aggressive can aggravate the condition and lead to inflammation, erythema, hemorrhage, and increased pain.
Post-operative rehabilitation has also shown to benefit patients with recent surgical resection of heterotopic ossification. The post-op management of HO is similar to pre-op treatment but much more emphasis is placed on edema control, scar management, and infection prevention. Calandruccio et al. outlined a rehabilitation protocol for patients who underwent surgical excision of heterotopic ossification of the elbow. The phases of rehab and goals for each phase are as follows:[3]
Phase I (Week 1)
Goals:
- Prevent infection
- Protect and decrease stress on surgical site
- Decrease pain
- Control and decrease edema
- ROM to 80% of affected joint
- Maintain ROM of joint proximal and distal to surgical site
Phase II (2-8 weeks)
Goals:
- Reduce pain
- Manage edema
- Encourage limited ADL performances
- Promote scar mobility and proper remodeling
- Promote full ROM of affected joint
- Encourage quality muscle contractions
Phase III (9-24 weeks)
Goals:
- Self-manage pain
- Prevent flare-up with functional activities
- Improve strength
- Improve ROM (if still limited)
- Return to previous levels of activity
Casavant and Hastings also provide great insight into the evaluation and management of heterotopic ossification in their article titled Heterotopic Ossification about the Elbow: A Therapist’s Guide to Evaluation and Management.[12]
Clinical Note: The two studies used above were primarily focused on rehabiliatation of the elbow secondary to heterotopic ossification. However, the goals and stages of the rehabilitation process can be used as a guide when treating at other sites.
Case Report[edit | edit source]
Title: Heterotopic Ossification Causing Radiculopathy after Lumbar Total Disc Arthroplasty
Key Words: Heterotopic Ossification, physical therapy, total disc arthroplasty, radiculopathy
Word Count: 520
Authors: Keith L. Jackson, Justin M. Hire, Jeremy M. Jacobs, Charles C. Key and John G. DeVine (modified by Morgan Blake and Hannah McCabe)
Abstract:
This case details the complications one man experienced due to the development of post-operative Heterotopic Ossification in his lumbar spine.
Introduction:
A total disc arthroplasty is a relatively new treatment strategy for lumbar discogenic back pain that has shown promising short- and intermediate-term results. Specific complications that can accompany total disc arthroplasty include vertebral body fracture, heterotopic ossification (HO), implant malposition, and early or late component extrusion. This case details a posterior component placement contributed to heterotopic bone growth within the spinal canal, causing neural impingement and radiculopathy and ultimately requiring component extraction, decompression, and lumbar arthrodesis.
Case Presentation:
- Subjective: 45 y/o male presents to the clinic with new onset R leg pain. He denies paresthesias or the loss of motor function, and he also denied a prior history of HO formation, trauma, or inflammatory arthritis. His PMH includes L5/S1 lumbar total disc arthroplasty 2 years ago for discogenic back pain. He reports that following surgery he had a significant reduction in his symptoms but developed this new pain about 6 months ago.
- Demographic Information: 45 years old, Male, works for UPS as a mail carrier
- Medical diagnosis: suspicion of HO
- Co-morbidities: HTN, DM
- Previous care/treatment: physical therapy (minimal improvements were noted)
- Self-report outcomes: Oswestry: 46% (Severe Disability)
- Objective: Physical examination revealed limited lumbar ROM in forward flexion due to R leg pain, positive single leg raise test, strength was 5/5 for all major muscle groups, intact sensation, and normal (2+) reflexes.
Patient was referred to his primary care provider who performed additional testing. Results of additional testing were:
- Radiographs demonstrated implant encroachment into the spinal canal with heterotopic bone formation outside the margins of the disc.
- Computed Tomography Myelogram revealed compression of the traversing nerve root secondary to the inferior endplate of the implant that resided posterior to the margin of the vertebral endplate as well as an associated posterior bone growth further into the canal.
Clinical Impression:
Patient demonstrates decreased lumbar ROM, with a history of trauma to the lumbar spine due to the lumbar disc arthroplasty. These factors and the positive imaging resulted in the patient being diagnosed with HO.
Intervention:
Patient elected to undergo surgical removal of his lumbar arthroplasty with fusion of L5/S1 and HO decompression. Patient returned to physical therapy post operatively for recovery.
Outcomes:
6 weeks post operatively patient reports complete relief of his radicular leg pain. Radiographs demonstrate a solid fusion without recurrence of HO formation.
Discussion:
As a physical therapist it is important to consider HO in your differential diagnosis when treating patients with a history of trauma or surgery. It is especially important to remember the signs and symptoms of HO when treating patients with limited ROM. We may be the first health professional to recognize the development of HO.
Acknowledgements:
Case adapted from “Heterotopic Ossification Causing Radiculopathy after Lumbar Total Disc Arthroplasty”[5]
Case Studies[edit | edit source]
Tibiofibular Syndesmosis and Ossification. Case Report: Sequelae of Ankle Sprains in an Adolescent Football Player.
Heterotopic Mesenteric Ossification ("intraabdominal myositis ossificans): A Case Report.
A Case of Psoas Ossification from the use of BMP-2 for Posterolateral Fusion at L4-L5.
- Brower RS, Vickroy NM. A case of psoas ossification from the use of BMP-2
for posterolateral fusion at L4–L5.Spine 2008; 18: 653-655.<span id="fck_dom_range_temp_1299789628126_629" />
Infrapatellar Heterotopic Ossification after Anterior Cruciate Ligament Reconstruction.
- Valencia H, Gavín C. Infrapatellar heterotopic ossification after anterior cruciate ligament reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy: Official Journal Of The ESSKA [serial on the Internet. (2007, Jan), [cited March 31, 2011]; 15(1): 39-42. Available from: MEDLINE.]
Heterotopic Ossification of the Ulnar Collateral Ligament: A Description of a Case in a Top Level Weightlifting Athlete.
- A Giombini, L Innocenzi, G Massazza, F Fagnani, et al. Heterotopic ossification of the ulnar collateral ligament: a description of a case in a top level weightlifting athlete. Journal of Sports Medicine and Physical Fitness. 2005 Sep 1;45(3): 370-80. In: ProQuest Medical Library [database on the Internet [cited 2011 Mar 31]. Available from: ProQuest; Document ID: 942792311]
Resources
[edit | edit source]
The Mystery of Heterotopic Ossification and How it Affected My Life
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Sun E, Hanyu-Deutmeyer AA. Heterotopic Ossification.Available: https://www.ncbi.nlm.nih.gov/books/NBK519029/(accessed 24.10.2021)
- ↑ Radiopedia HO Availasble: https://radiopaedia.org/articles/heterotopic-ossification(accessed 24.10.2021)
- ↑ 3.0 3.1 3.2 3.3 Hsu JE, Keenan MA. Current review of heterotopic ossification. UPOJ 2010; 20: 126-130.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic Ossification Revisited. Orthopedics. 2011Jan;34(3):177.
- ↑ 5.0 5.1 Dalury DF, Jiranek WA. The incidence of heterotopic ossification after total knee arthroplasty. Journal of Arthroplasty 2004; 19: 447-457.
- ↑ Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. Jour of Nuclear Medicine 2002; 43: 346-353.
- ↑ Orthobullets HO Available: https://www.orthobullets.com/pathology/8044/heterotopic-ossification(accessed 24.10.2021)
- ↑ 8.0 8.1 8.2 8.3 8.4 Firoozabadi R, Alton T, Sagi HC. Heterotopic Ossification in Acetabular Fracture Surgery. Journal of the American Academy of Orthopaedic Surgeons. 2017;25(2):117–24.
- ↑ 9.0 9.1 9.2 9.3 Bossche LV, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005; 37: 129-136.5. Pape HC et al. Current concepts in the development of hetetrotopic ossification. Journ Bone and Joint Surg 2004; 86: 783-787.
- ↑ 10.0 10.1 Pape HC et al. Current concepts in the development of hetetrotopic ossification. Journ Bone and Joint Surg 2004; 86: 783-787.
- ↑ McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol 2005; 34: 609-619.
- ↑ 12.0 12.1 12.2 Foruria AM, Augustin S, Morrey BF, Sanchez-Sotelo Joaquin. Heterotopic Ossification After Surgery for Fractures and Fractures-Dislocations Involving the Proximal Aspect of the Radius or Ulna. The Journal of Bone and Joint Surgery, Incorporated. 2015May15;95-A(10):e66(1)-e66(7).