Transcranial Magnetic Stimulation
Original Editor - Riccardo Ugrin
Top Contributors - Riccardo Ugrin, Angeliki Chorti, Kevin Parcetich, Oyemi Sillo, Kim Jackson, Candace Goh, Lucinda hampton and Sehriban Ozmen
Introduction[edit | edit source]
Transcranial Magnetic Stimulation (TMS) is a non invasive brain stimulation modality based on the principles of electromagnetic induction. A coil is placed on the scalp and either single (sTMS) or repeated (rTMS) magnetic pulses are delivered through a device to the brain; the frequency, intensity, direction, duration and interval times of pulses may vary. [1]
The exact mechanism of action is still unclear, but current evidence points toward its role in causing long-term neuromodulatory effects in certain brain areas. [2]
There is a sufficient body of evidence to accept: [3]
- Level A (definite efficacy) for:
- An analgesic effect of high frequency (HF) rTMS of the primary motor cortex (M1) contralateral to the pain
- The efficacy of HF rTMS of the left dorsolateral prefrontal cortex (DLPFC) in depression
- The efficacy of rTMS in the treatment of unipolar depression for both HF rTMS of the left DLPFC and LF rTMS of the right DLPFC.
- Level B recommendation (probable efficacy) is proposed for:
- The antidepressant effect of low-frequency (LF) rTMS of the right DLPFC,
- The negative symptoms of schizophrenia HF-rTMS of the left DLPFC ,
- Motor recovery for chronic stroke patients with LF-rTMS of contralesional M1
- Level C (possible efficacy) for the effect of LF rTMS of the left temporoparietal cortex in tinnitus and auditory hallucinations

Historical Background[edit | edit source]
Throughout history, brain stimulation techniques such as TMS have proved to be powerful tools for investigating neurophysiology as well as for mapping and modulating neural circuitry. [1] First emerging as a potential tool for noninvasive neuronal stimulation in the early 20th century,[5] repetitive transcranial magnetic stimulation (rTMS) has undergone multiple stages of development.
In 1985, Anthony Barker invented the first modern-day TMS device as a way to study electrophysiology.[6] Barker’s original research was based on single-pulse TMS where a single stimulus was delivered to a specific brain region. The easiest muscles to activate are those in the hand and distal upper limb due, in part, to the convenient location of the hand motor area in the central convexity of the brain and, in part, to physiology; these muscles have the purest component of corticospinal innervation from the opposite hemisphere. These studies demonstrated in fact that TMS could induce muscle movements in the hand when applied to the primary motor cortex (M1).
Expanding on this, the technology developed to allow a device to deliver multiple stimuli over a short period of time, to have lasting effects on cortical excitability that persisted beyond the actual stimulus delivery.
Given the ability of this treatment to modulate cortical activity in a focal way, focus was soon placed on the use of this technique to potentially ameliorate neuropsychiatric disorders, with the earliest studies attempting to treat depression.
In 1995, George et al.[7] used rTMS to target specific prefrontal brain regions thought to be involved in the etiology or pathophysiology of major depression in an open-label study. Although most of the research has supported the antidepressant properties of rTMS, the degree of clinical benefit has been variable and, in some cases, marginal. However, a clear trend toward more robust effects has been seen as both stimulation technique (e.g., dose, coil placement, and course duration) and research quality (e.g., better sham stimulation and larger sample sizes) improve. rTMS has become a recogniSed, accepted, and clinically available therapeutic intervention. Since these first studies, rTMS has been explored as a therapeutic intervention for neuropsychiatric disorders such as treatment-resistant depression, numerous clinical trials of rTMS for the treatment of depression (and other psychiatric disorders) have been conducted. [8][9][10]
In October 2008, an rTMS device was approved for use by the US Food and Drug Administration (FDA) for patients with major depression who have not responded to at least one antidepressant medication in their present episode.
With the advent of more advanced structural and functional neuroimaging over the past several decades, the specifics of this network have become better understood. [11]
Functioning[edit | edit source]
The equipment uses a high current pulse generator able to produce a discharge current of several thousand amperes that flows through a stimulating coil, generating a brief magnetic pulse with field strengths up to several Teslas. This magnetic pulse, by the principles of electromagnetism, produces secondary electric fields in the opposite direction to the field generated by the coil. [12][13][14][15] If the coil is placed on the head of a subject, the magnetic field thus created undergoes little attenuation by extracerebral tissues (scalp, cranial bone, meninges, and cerebrospinal fluid layer) and is able to induce an electrical field sufficient to depolarize superficial axons and to activate neural networks in the cortex.
The extent of action of the current density generated into the brain depends on many physical and biological parameters, such as the type and orientation of coil, the distance between the coil and the brain, the magnetic pulse waveform, the intensity, frequency and pattern of stimulation, and the respective orientation into the brain of the current lines and the excitable neural elements.
The simplest and most easily quantified measure of muscle contraction is motor threshold, that is, the intensity of stimulation that produces the smallest reproducible activation of the tested muscle. Reduced motor threshold is considered to represent a state of greater cortical excitability, and higher threshold a state of lower excitability.
Types of TMS[edit | edit source]
- Single-pulse TMS (spTMS) can be used to simply stimulate a given area while recording the output and is commonly used diagnostically and in research where an area such as the motor cortex is stimulated and a motor response can be recorded from muscles of the body via electromyography. [14][15] This allows for an electrophysical investigation of cortical response and human cortex connectivity. [16]
- Paired-pulse TMS (ppTMS) can be used to assess the effect of a preceding stimulus on a secondary stimulus. While this technique is also primarily used in research, it allows for the assessment of one brain region on another. For example, a TMS pulse delivered to the motor cortex of one hemisphere of the brain 10ms prior to a TMS pulse delivered over the opposite motor cortex results in an inhibitory effect in motor output to the arms, showing firing patterns that allow for unimanual control of the upper limbs. [17][18][19]
- Repetitive TMS (rTMS) techniques involve stringing a large number of consecutive TMS pulses together in rapid succession. This method is used both in research and clinically as it can produce changes in cortical activity that last beyond the duration of the TMS protocol. With some reports demonstrating excitability changes persisting for a number of hours. The rate of pulse delivery appears to dictate the effect of the rTMS protocol, whereas those that deliver pulses at rates of >5Hz (high frequency rTMS) tend to produce excitatory effects and those delivered at rates of < 1Hz (low frequency rTMS) tend to produce inhibitory effects in the brain. These rTMS techniques have been approved as a treatment modality for those with non-responsive major depression disorder (MDD) in Canada. While the use of rTMS has not yet been approved for clinical use in the treatment of movement disorders such as stroke, spinal cord injury, and PD, the scientific literature suggests that it may provide some benefit to both motor and cognitive symptoms in these populations. [20][21][22][23]
Frequency[edit | edit source]
- Changes in motor-evoked potentials suggest that rTMS alters cortical excitability in a frequency-dependent manner. [13] From the results obtained in different studies based on MEP measurement in healthy subjects, some form of consensus appeared to consider low-frequency (LF) stimulation (≤1Hz) as “inhibitory” and high-frequency (HF) stimulation (≥5Hz) as “excitatory”. The mechanisms by which these neuroadaptations occur remain unclear, but some have speculated that rTMS induces a Hebbian plasticity that resembles long-term potentiation (LTP) or long-term depression (LTD).
- HF rTMS might be in fact the result of a decrease of gamma-amminobutyric acid (GABA)-mediated intracortical inhibition (hence inhibition of inhibition), rather than a direct enhancement of motor cortex excitability. For high-frequency TMS, a cooling system is required to prevent overheating of the electromagnet with repeated stimulation.
- LF rTMS can enhance the net inhibitory corticospinal control, probably via GABA-B transmission.
- One of the most popular protocol is the “theta burst stimulation” delivered as a continuous (cTBS) or intermittent (iTBS) train, the former protocol being “inhibitory” and the latter being “excitatory”. [20]
- However, this dichotomy is not entirely clear, and it has been shown that both HF and LF rTMS may have mixed excitatory and inhibitory effects. [24] Even when the effect on the motor cortex appears specific, doubling the duration of stimulation, for example, can reverse the outcome from inhibition to excitation and vice versa. [25]
Coils[edit | edit source]
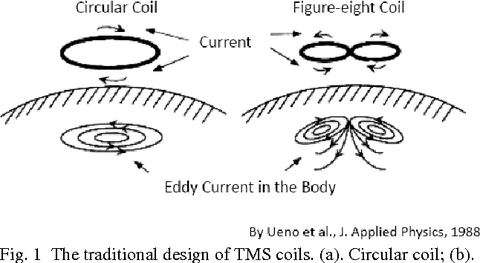
Since TMS was first introduced, researchers and engineers have proposed a striking variety of coil configurations, all intended to optimally focus the induced electric current within the brain. The use of larger coils stimulate wider and deeper volumes of the underlying brain. However, this may be a disadvantage if the target can be precisely localised. A study [26] reported that the final output of these varied designs could be reduced to two fundamental arrangements: a round coil and a double (figure-8) coil.
- Round coils do have advantages, including simple construction, straightforward heat dissipation, stable head contact, and relatively good penetration beneath the scalp surface. However, the near impossibility of aiming the coils toward a single brain region limits their utility in most applications. [1]
 Transcranial Magnetic Stimulation Coil System. [27]
Transcranial Magnetic Stimulation Coil System. [27] - Double (figure-8) coils consist of two round coils placed side by side (forming a figure-8 shape) which somewhat improves efficiency and penetration. [28]
- A recent technological advance in TMS is the H-shaped coil, which stimulates deeper than conventional figure-8 coils. Recent studies have addressed the safety of these coils in small groups of patients with bipolar depression.[29]
Intensity[edit | edit source]
Stimulation intensity is usually expressed as a percentage of rest motor threshold (RMT), which is the minimum intensity required to elicit an electromyographic (EMG) response (motor-evoked potential [MEP]) of at least 50 μV, with a probability of 50% in a hand muscle at rest. [30] RMT can also be determined by observing clinical motor responses (finger movement) rather than recording MEP.
Contraindications[edit | edit source]
The only absolute contraindication of TMS is the presence of ferromagnetic material or implanted devices in close contact with the coil (less than 2 cm) because of the risk of displacement or malfunction.
Relative contraindications that require specific justification or indication for rTMS.are:
- cochlear implants or other intracranially implanted hardware,
- implanted cortical stimulation or DBS systems are present,
- cortical TMS may be considered in cases of cardiac pacemaker or vagus nerve or spinal cord stimulation if material thicker than 10 cm is placed over the implanted generator,
- pregnant women,
- children (age >2 years),
- patients with hearing disorders require specific justification or indication for rTMS,
- personal history of epilepsy,
- focal cerebral lesion,
- drug intake or removal that lowers the seizure threshold,
- sleep deprivation
TMS for Depression[edit | edit source]
Some of the most commonly implicated brain regions include the dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex, orbitofrontal cortex, cingulate gyrus (including dorsal anterior, perigenual, subgenual, and posterior subdivisions), insular cortex, medial temporal lobe regions (hippocampus, parahippocampus, and amygdala), parietal cortex, thalamus, midbrain structures (including dorsal and ventral striatum, hypothalamus), and brain stem regions. Abnormalities in these various regions have been identified in depressed patients versus healthy controls. It was found a correlation between cerebral metabolic activity and TMS effectiveness. This was validated by the results of TMS treatment in non-responders who exhibited hypoperfusion in the frontal cortex.
High-frequency stimulation of the left DLPFC alleviates depressive symptoms, whereas low-frequency cortical stimulation of the right DLPFC helps to relieve the symptoms of both depression and anxiety. In fact, this result is related to the decrease in synaptic strength observed in depression and may be attributed to the fact that fast rTMS (> 10 Hz) results in neuronal excitation while slow rTMS (< 1 Hz) has the reverse effects. [31]
Study shows as daily sessions of TMS over the DLPFC increase cortical activity and reduce activity in distant areas. Neuronal physiology that responds to TMS is of central importance, as repetitive stimulations increase synaptic plasticity, causing it to last longer even after stimulation ceases. Many randomised clinical trials have shown that daily TMS of the left prefrontal cortex was effective in treating depressive mood symptoms. [32][33]
Some studies demonstrated long-term benefits from TMS followed by pharmacotherapy or no medication in 6 months follow-up. [34][35] TMS demonstrates a statistically and clinically meaningful durability of acute benefit also over 12 months of follow-up. However all these results were observed under a pragmatic regimen of continuation antidepressant medication and access to TMS retreatment for symptom recurrence. [36]
TMS for Neurological Disease[edit | edit source]
The most recent guidelines [3] for TMS in neurological disease show the following indications:
- Despite the amount of published work, the data in the literature is still too limited to support any recommendation regarding therapeutic use of rTMS in:
- Cerebellar ataxia
- Myoclonia
- Huntington’s disease
- Amyotrophic lateral sclerosis
- Multiple sclerosis
- Alzheimer’s disease
- Tinnitus, it appears that single LF rTMS sessions or repeated rTMS sessions, when delivered to the auditory cortex contralateral to the tinnitus side, justify a Level C recommendation.[37]
- Anxiety disorders
- In contrast, there are much more convincing data regarding:
- Parkinson's Disease; for more information please see Repetitive Transcranial Magnetic Stimulation Treatment For Parkinson's
- Dystonia (in particular writer’s cramp),
- Essential tremor,
- Tourette’s syndrome.
- Epilepsy
- Stroke patients:
- There are some indication to efficacy in motor abilities improve in stroke patient: excitability-increasing HF rTMS of ipsilesional M1 or excitability-decreasing LF rTMS of contralesional M1 have Levels B or C recommendation. It must be emphasized that the therapeutic value of either modality of stimulation remains to be determined with respect to the phase of stroke recovery (acute or sub-acute vs. chronic).
- Possible efficacy also exists (Level C recommendation) for the use of repeated trains of cTBS delivered to the posterior parietal cortex of the contralesional left hemisphere in hemispatial neglect. For more information please see Unilateral Neglect.
- Confirmation of promising results are expected very soon regarding the use of LF rTMS of the pars triangularis of the IFG of the contralesional right hemisphere in nonfluent Broca's aphasia
- Depression: the efficacy of HF rTMS of the left DLPFC in depression is definite, with a Level A recommendation.
- unipolar or bipolar depression: the efficacy of rTMS in the treatment of unipolar depression with a Level A recommendation (“definite efficacy”) for both HF rTMS of the left DLPFC and LF rTMS of the right DLPFC.
Transcranial Magnetic Stimulation: A New Treatment Approach for Psychiatric Disorders [38]
TMS for Neuropathic Pain[edit | edit source]
Several brain areas, including the hypothalamus, amygdala, thalamus, somatosensory cortex, insula, anterior cingulate cortex, and prefrontal cortex, participate in the experience of pain. Moreover, some patients with chronic pain do not respond to various conventional treatments, including drugs, injections with anesthetics and corticosteroids, and behavioural therapies. rTMS is postulated to induce alterations in the activity of cortical and subcortical brain structures that are related to pain modulation and processing, including the orbitofrontal cortices, medial thalamus, anterior cingulate, and periaqueductal gray matter. Specifically, rTMS is known to alter neuronal activities in the periaqueductal gray matter, which is related to pain processing. [39]
- Most studies oriented the coils of the rTMS to the posteroanterior zone of the motor cortex (M1). Patients had best results with 10 sessions of HF rTMS on M1.
- Good results were obtained with the coil oriented on the right DLPFC. Infact the stimulation of the right DLPFC was also effective in rapid-onset pain relief.
- No results among patients with chronic neuropathic pain after stroke or spinal cord injury who received deep stimulation of the anterior cingulated cortex (ACC) or the posterior superior insula (PSI) compared with sham rTMS. [40]
TMS for Migraine[edit | edit source]
Studies have reported that the mechanisms of migraine are likely related to neural and vascular causes, including cerebral cell hyperexcitability, sensitisation of the trigeminovascular pathway, genetics, and environmental factors. [41] As it shown above RTMS has the potential to increase the activity of cortical structures that are involved in pain control or decrease cortical excitability. It was also found that plasma β endorphin levels were lower in patients with migraine than those in patients without migraine and that HF rTMS increased β endorphin levels. [42][43]
HF rTMS were applied at the left M1 and at the left DLPFC with good results for headache at the 4-week assessment after treatment. [44][45]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Holtzheimer P, McDonald W. (Eds.), A Clinical Guide to Transcranial Magnetic Stimulation. Oxford, UK: Oxford University Press. 2014.
- ↑ Chail A, Saini R, Bhat P, Srivastava K, Chauhan V. Transcranial magnetic stimulation: A review of its evolution and current applications. Ind Psychiatry J. 2018 Jul-Dec;27(2):172-180.
- ↑ 3.0 3.1 Lefaucheur J, André-Obadia N, Antal A, Ayache S, Baeken C, Benninger D, Cantello R, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović S, Hummel F, Jääskeläinen S, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini P, Rothwell J, Schönfeldt-Lecuona C, Siebner H, Slotema C, Stagg C, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS)". Clin Neurophysiol. 2014 Nov;125(11):2150-2206.
- ↑ Sheng G, Wang J, Tang H, Xiao X, Wu W. Transcranial Magnetic Stimulation Coil System.(2012)
- ↑ Thompson S. A physiological effect of an alternating magnetic field. Proceedings of the Royal Society B: Biological Sciences, 1910; 82(557): 396–398.
- ↑ Barker A, Freeston I, Jalinous R, Merton P, Morton H. Magnetic stimulation of the human brain. J Physiol. 1985; 369: 1–3.
- ↑ George M, Wassermann E, Williams W, Callahan A, Ketter T, Basser P, Hallett M, Post R. Daily repetitive transcranial magnetic stimulatioan (rTMS) improves mood in depression. Neuroreport,1995; 6(14): 1853–1856.
- ↑ Hoflich G, Kasper S, Hufnagel A, Ruhrmann S, Moller H. Application of transcranial magnetic stimulation in treatment of drug-resistant major depression—A report of two cases. Hum Psychopharmacol. 1993; 8: 361–65.
- ↑ Kolbinger H, Hoflich G, Hufnagel A, Moller H, Kasper S. Transcranial magnetic stimulation (TMS) in the treatment of major depression - a pilot study. Hum Psychopharmacol. 1995; 10(4): 305–10.
- ↑ Pascual-Leone A, Rubio B, Pallardo F, Catala M. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996; 348(9022): 233–37.
- ↑ Drevets W, Price J, Furey M. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008; 213(1-2): 93–118.
- ↑ Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985 May 11;1(8437):1106-7.
- ↑ 13.0 13.1 Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147-50.
- ↑ 14.0 14.1 Hallett M. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007;55:187-99.
- ↑ 15.0 15.1 Siebner H, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1-16.
- ↑ Noda Y, Barr MS, Zomorrodi R, Cash RFH, Lioumis P, Chen R, Daskalakis ZJ, Blumberger DM. Single-Pulse Transcranial Magnetic Stimulation-Evoked Potential Amplitudes and Latencies in the Motor and Dorsolateral Prefrontal Cortex among Young, Older Healthy Participants, and Schizophrenia Patients. J Pers Med. 2021 Jan 17;11(1):54.
- ↑ Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1-10.
- ↑ Ni Z, Gunraj C, Nelson A, Yeh I, Castillo G, Hoque T, Chen R. Two Phases of Interhemispheric Inhibition between Motor Related Cortical Areas and the Primary Motor Cortex in Human. Cerebr Cortex. 2009;19:1654-65.
- ↑ Ferbert A, Priori A, Rothwell J, Day B, Colebatch J, Marsden C. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525-46.
- ↑ 20.0 20.1 Huang Y-Z, Edwards MJ, Rounis E, Bhatia K, Rothwell J. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005; 45:201-6.
- ↑ Le Q, Qu Y, Tao Y, Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am J Phys Med Rehabil. 2014 May 1;93(5):422-30.
- ↑ Tazoe T, Perez MA. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. Arch Phys Med Rehabil. 2015 Apr;96(4 Suppl):S145-55.
- ↑ Goodwill AM, Lum JAG, Hendy AM, Muthalib M, Johnson L, Albein-Urios N, Teo WP. Using non-invasive transcranial stimulation to improve motor and cognitive function in Parkinson's disease: a systematic review and meta-analysis. Sci Rep. 2017 Nov 1;7(1):14840.
- ↑ Houdayer E, Degardin A, Cassim F, Bocquillon P, Derambure P, Devanne H. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp Brain Res. 2008 May;187(2):207-17.
- ↑ Gamboa O, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst aftereffect with prolonged stimulation. Exp Brain Res 2010;204:181-7.
- ↑ Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013 Jan;6(1):1-13.
- ↑ Sheng G, Wang J, Tang H, Xiao X, Wu W. Transcranial Magnetic Stimulation Coil System.(2012). Available from: https://www.semanticscholar.org/paper/Transcranial-Magnetic-Stimulation-Coil-System-Ge-Wang/384a83b4a35699f51d7a9d55e74489034883c9b8 [accessed 28-1-24]
- ↑ Lontis E, Voigt M, Struijk J. Focality assessment in transcranial magnetic stimulation with double and cone coils. J Clin Neurophysiol. 2006 Oct;23(5):462-71.
- ↑ Harel EV, Zangen A, Roth Y, Reti I, Braw Y, Levkovitz Y. H-coil repetitive transcranial magnetic stimulation for the treatment of bipolar depression: an add-on, safety and feasibility study. World J Biol Psychiatry. 2011 Mar;12(2):119-26.
- ↑ Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994 Aug;91(2):79-92.
- ↑ Nahas Z, Teneback CC, Kozel A, Speer AM, DeBrux C, Molloy M, Stallings L, Spicer KM, Arana G, Bohning DE, Risch SC, George MS. Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. J Neuropsychiatry Clin Neurosci. 2001 Fall;13(4):459-70.
- ↑ Rizvi S, Khan AM. Use of Transcranial Magnetic Stimulation for Depression. Cureus. 2019;11(5):e4736.
- ↑ George M, Taylor J, Short E. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013 Jan;26(1):13-8.
- ↑ Mantovani A, Pavlicova M, Avery D, Nahas Z, McDonald W, Wajdik C, Holtzheimer P 3rd, George M, Sackeim H, Lisanby S. Long-term efficacy of repeated daily prefrontal transcranial magnetic stimulation (TMS) in treatment-resistant depression. Depress Anxiety. 2012 Oct;29(10):883-90.
- ↑ Solvason H, Husain M, Fitzgerald P, Rosenquist P, McCall W, Kimball J, Gilmer W, Demitrack M, Lisanby S. Improvement in quality of life with left prefrontal transcranial magnetic stimulation in patients with pharmacoresistant major depression: acute and six month outcomes. Brain Stimul. 2014 Mar-Apr;7(2):219-25.
- ↑ Dunner D, Aaronson S, Sackeim H, Janicak P, Carpenter L, Boyadjis T, Brock D, Bonneh-Barkay D, Cook I, Lanocha K, Solvason H, Demitrack M. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014 Dec;75(12):1394-401.
- ↑ Anders M, Dvorakova J, Rathova L, Havrankova P, Pelcova P, Vaneckova M, Jech R, Holcat M, Seidl Z, Raboch J. Efficacy of repetitive transcranial magnetic stimulation for the treatment of refractory chronic tinnitus: a randomized, placebo controlled study. Neuro Endocrinol Lett. 2010;31(2):238-49.
- ↑ Barbour T. Transcranial Magnetic Stimulation: A New Treatment Approach for Psychiatric Disorders. Available from: http://www.youtube.com/watch?v=So-boB9niXQ [accessed 30/1/2024]
- ↑ Yang S, Chang M. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front Neurol. 2020 Feb 18;11:114.
- ↑ Galhardoni R, Aparecida da Silva V, Garcia-Larrea L, Dale C, Baptista AF, Barbosa LM, Menezes LMB, de Siqueira SRDT, Valério F, Rosi J Jr, de Lima Rodrigues AL, Reis Mendes Fernandes DT, Lorencini Selingardi PM, Marcolin MA, Duran FLS, Ono CR, Lucato LT, Fernandes AMBL, da Silva FEF, Yeng LT, Brunoni AR, Buchpiguel CA, Teixeira MJ, Ciampi de Andrade D. Insular and anterior cingulate cortex deep stimulation for central neuropathic pain: disassembling the percept of pain. Neurology. 2019; 92:e2165–75
- ↑ Lan L, Zhang X, Li X, Rong X, Peng Y. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J Headache Pain. 2017; 18(1):86.
- ↑ Misra UK, Kalita J, Tripathi GM, Bhoi SK. Is beta endorphin related to migraine headache and its relief? Cephalalgia. 2013; 33:316–22.
- ↑ Misra UK, Kalita J, Tripathi G, Bhoi SK. Role of beta endorphin in pain relief following high rate repetitive transcranial magnetic stimulation in migraine. Brain Stimul. 2017; 10(3):618–23.
- ↑ Leung A, Shukla S, Fallah A, Song D, Lin L, Golshan S, Tsai A, Jak A, Polston G, Lee R. Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation. 2016; 19(2):133–41.
- ↑ Leung A, Metzger-Smith V, He Y, Cordero J, Ehlert B, Song D, Lin L, Shahrokh G, Tsai A, Vaninetti M, Rutledge T, Polston G, Sheu R, Lee R. Left Dorsolateral prefrontal cortex rTMS in alleviating MTBI related headaches and depressive symptoms. Neuromodulation. 2018; 21(4):390–401.






