Alzheimer's Disease: Difference between revisions
No edit summary |
No edit summary |
||
| Line 89: | Line 89: | ||
{{#ev:youtube|chgshB6LCyc}} | {{#ev:youtube|chgshB6LCyc}} | ||
== Medications == | == Medications == | ||
Below is a list of some commonly used medications use in the treatments of the symptoms of Alzheimer's. There is also the use of other treatments such as antioxidants, anti-inflammatory agents, and estrogen replacement therapy in women to prevent or delay the onset of the disease.<ref>Porth C. Pathopysiology Concepts of Altered Health States. Philadelphia PA: Lippincott and Wilkins; 2005.</ref><ref>Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.</ref> | |||
* Cholinesterase inhibitors e.g. donepezil | |||
* | * Partial NMDA receptor antagonists | ||
* | * Medications for behavioural symptoms | ||
* | * Antidepressants | ||
* | * Anxiolytics | ||
* | * Antiparkinsonian (movement symptoms) | ||
* | * Anticonvulsants/sedatives (behavioural)<ref name=":1" /> | ||
== Diagnosis == | == Diagnosis == | ||
| Line 119: | Line 109: | ||
* EEG shows a slowing with no focal features, again nonspecific. | * EEG shows a slowing with no focal features, again nonspecific. | ||
* The most reliable tool for finding mild cognitive impairment in early disease is neuropsychological testing. including a psychiatric evaluation to see if depression or another mental health condition is causing or contributing to a person's symptoms. | * The most reliable tool for finding mild cognitive impairment in early disease is neuropsychological testing. including a psychiatric evaluation to see if depression or another mental health condition is causing or contributing to a person's symptoms. | ||
* | * CT reveals the characteristic cortical atrophy, however MRI is the favoured modality as it shows greater detail. Molecular imaging with PET is gaining use in the diagnosis of Alzheimer disease. | ||
* Genetic Testing: | * Genetic Testing: Inheriting a single copy of the ApoE gene, encoding for apolipoprotein E, increases the chances of developing Alzheimer disease three times, whilst inheriting both copies increases one's risk eightfold.<ref name=":1" /> | ||
== Screening Tools == | |||
Objective tools have been validated in the practice of physical therapy in order to screen for AD such as the Mini-Cog, [[Mini-Mental State Examination|Mini-Mental State Exam]] (MMSE), Clock-Drawing, & Neurobehavioral Cognitive Status Exam. A pilot study developed a study protocol aimed at aiding the early detection of dementia disorders using the Timed Up-and-Go (TUG) test with the verbal task of naming different animals<ref>Cedervall Y, Stenberg AM, Åhman HB, Giedraitis V, Tinmark F, Berglund L, Halvorsen K, Ingelsson M, Rosendahl E, Åberg AC. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7084863/ Timed Up-and-Go Dual-Task Testing in the Assessment of Cognitive Function: A Mixed Methods Observational Study for Development of the UDDGait Protocol.] International journal of environmental research and public health. 2020 Jan;17(5):1715.</ref>. | Objective tools have been validated in the practice of physical therapy in order to screen for AD such as the Mini-Cog, [[Mini-Mental State Examination|Mini-Mental State Exam]] (MMSE), Clock-Drawing, & Neurobehavioral Cognitive Status Exam. A pilot study developed a study protocol aimed at aiding the early detection of dementia disorders using the Timed Up-and-Go (TUG) test with the verbal task of naming different animals<ref>Cedervall Y, Stenberg AM, Åhman HB, Giedraitis V, Tinmark F, Berglund L, Halvorsen K, Ingelsson M, Rosendahl E, Åberg AC. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7084863/ Timed Up-and-Go Dual-Task Testing in the Assessment of Cognitive Function: A Mixed Methods Observational Study for Development of the UDDGait Protocol.] International journal of environmental research and public health. 2020 Jan;17(5):1715.</ref>. | ||
Revision as of 07:03, 16 January 2023
Original Editors - Students from Bellarmine University's Pathophysiology of Complex Patient Problems project. Top Contributors - Josie Little, Stephanie Schwebler, Laura Ritchie, Admin, Lucinda hampton, Elaine Lonnemann, Kim Jackson, Hayaa Yousri, Emily Pollom, Dave Pariser, Vidya Acharya, Kirenga Bamurange Liliane, Nikhil Benhur Abburi, Lauren Lopez, 127.0.0.1, Evan Thomas, Shaimaa Eldib, Joseph Ayotunde Aderonmu, Naomi O'Reilly, WikiSysop, Tolulope Adeniji, Safiya Naz and Wendy Walker
Introduction[edit | edit source]
Alzheimer's Disease (AD) is the most common cause of dementia worldwide[1] It is a neurodegenerative disorder. The primary known risk factor for the disease is ageing, but AD is not a normal part of ageing. Alzheimer’s Disease is progressive so symptoms will worsen with time.[2] AD results from the of accumulation and deposition of cerebral amyloid-β (Aβ) cerebral amyloid. There is currently no cure for the disease, but treatments are available to slow down the progression[3][2].
Epidemiology[edit | edit source]
Alzheimer disease is the most prevelent cause of dementia, accounting for 60-80% of all dementias. The prevalence is closely linked to age, >1% of 60-64-year-olds having the condition rising to 20-40%in the over 85-90 age bracket.[3]
Pathology[edit | edit source]
Alzheimer disease is distinguished accumulation within the brain of cerebral amyloid-β (Aβ or Abeta). These go on to form neuritic plaques, neurofibrillary tangles and indue time progressive neurone loss.
Cerebral amyloid-β deposits occur predominantly
- Entorhinal cortex in the hippocampus
- Association areas of the neocortex
- Posterior cingulate and precuneus,
- Limbic cortex
The fundamental reason for the accumulation of neuritic plaques and neurofibrillary tangles is not as yet understood. Evidence partially points to chronic inflammation having a role. This inflammatory state leads to prolonged activation of microglial cells which causes inflammatory mediators to be released resulting in neuronal damage and amyloid-induced neurodegeneration.[3]
Etiology[edit | edit source]
Alzheimer's disease is a progressive neurodegenerative disease caused by nerve cell death. Genetics is a factor in some cases of early and late-onset Alzheimer's disease. Several risk factors have been associated with Alzheimer's disease including[4]:
- Advancing age, >85 y/o risk increases by nearly 50%[5]
- Direct family member with the disease (mother, father, brother or sister)
- Apolipoprotein E-e4 (APOE4) carries the strongest risk of developing Alzheimer’s Disease (a genetic mutation of APOE) [6]
- Traumatic brain injury
- Deterministic genes have a direct cause of early-onset AD, however, they only account for less than 5% of cases: amyloid precursor protein (APP), presenilin-1 (PS-1), presenilin (PS-2) [7]
- Trisomy 21
- Cardiovascular risk factors: mid-life obesity, mid-life hypertension, hyperlipidemia, diabetes mellitus[8]
As well as the genetic and environmental factors above, the age when clinical signs show is affected by by socioeconomic factors:
- Formal education
- Income
- Occupational status
- Social network and family support[3]
People with higher function/supports prior to diagnosis are able to compensate for early disease changes more effectively and present later. When these people present, they tend to have more marked morphological changes on imaging.[3]
The Possible Protective Factors[edit | edit source]
The factors below have been suggested to reduce the risk of developing Alzheimer's disease:[9]
- Apolipoprotein E2 gene
- Regular fish consumption
- Regular consumption of omega - 3 fatty acids
- Years of higher education
- Regular exercise due to cardiovascular benefits increasing oxygen & blood to the brain
- Diets low in sugar and saturated fats
- Prevention of head trauma & falls
- Nonsteroidal anti-inflammatory drug therapy
- Moderate Alcohol intake
- Adequate intake of vitamins C,E, B6, and B12, and folate.
Clinical Presentation[edit | edit source]
The progression of Alzheimer's Disease is continuous and generally does not fluctuate or improve. Often times the early symptoms can be missed or overlooked because they can be misinterpreted as signs of the natural ageing process[10]. The typical patient with Alzheimer disease will present initially with decreased ability to form/retain new memories. With time (often years), cognitive deficeits progresses, with eventual problems with attentional and executive processes, semantic memory, and visuoperceptual abilities. Mental health problems affect almost all patients eventually, including apathy, depression, anxiety, aggression/agitation, and psychosis (delusions and hallucinations).[3]
Stages of Alzheimer's Disease
Alzheimer's disease may progress through the following stages as follows[11][12]:
Mild Alzheimer’s Disease (Early Stage)
- May Function Independently: may drive, work or maybe apart of social activities
- Memory Lapses: familiar words, location of objects, names of new people, recently read material
- Difficulties noticed by family, friends and doctors: challenges performing activities at home or work, difficulty planning
- Lack of spontaneity
- Subtle personality changes
- Disorientation to time and date
Moderate Alzheimer’s Disease (Middle Stage)
- Longest stage may last for years
- Personality changes: moody or withdrawn, suspicious, delusions, compulsive, repetitive behavior
- Increased memory loss: forgetfulness regarding personal history, unable to recall address, phone number, or high school they graduated from
- Decreased independence: trouble controlling bowel and bladder, increased risk of wandering or becoming lost, dependence with choosing appropriate clothes for event or season, increased Confusion
- Impaired cognition and abstract thinking
- Restlessness and agitation
- Wandering, "sundown syndrome"
- Inability to carry out activities of daily living
- Impaired judgement
- Inappropriate social behavior
- Lack of insight, abstract thinking
- Repetitive behavior
- Voracious appetite
Severe Alzheimer’s Disease (Late Stage)
- Decreased response to the environment: decreased ability to communicate and may speak in small phrases, decreased awareness of experiences & surroundings
- Dependence on caregiver: decreased physical functioning: walking, sitting & swallowing; increased vulnerability to infections, incontinence
- Emaciation, indifference to food
- Inability to communicate
- Urinary and fecal incontinence
- Seizures
Medications[edit | edit source]
Below is a list of some commonly used medications use in the treatments of the symptoms of Alzheimer's. There is also the use of other treatments such as antioxidants, anti-inflammatory agents, and estrogen replacement therapy in women to prevent or delay the onset of the disease.[13][14]
- Cholinesterase inhibitors e.g. donepezil
- Partial NMDA receptor antagonists
- Medications for behavioural symptoms
- Antidepressants
- Anxiolytics
- Antiparkinsonian (movement symptoms)
- Anticonvulsants/sedatives (behavioural)[3]
Diagnosis[edit | edit source]
Currently, the diagnosis of Alzheimer’s relies primarily on signs and symptoms of mental decline. Routine laboratory tests show no specific abnormality. CT brain reveal cerebral atrophy and widened the third ventricle, a nonspecific finding as these abnormalities are also present in other illnesses and people with normal age-related changes. [15]
Tests include:
- Cerebrospinal fluid (CSF) analysis for low beta-amyloid 42 and elevated tau helps at the pre-clinical stage.
- EEG shows a slowing with no focal features, again nonspecific.
- The most reliable tool for finding mild cognitive impairment in early disease is neuropsychological testing. including a psychiatric evaluation to see if depression or another mental health condition is causing or contributing to a person's symptoms.
- CT reveals the characteristic cortical atrophy, however MRI is the favoured modality as it shows greater detail. Molecular imaging with PET is gaining use in the diagnosis of Alzheimer disease.
- Genetic Testing: Inheriting a single copy of the ApoE gene, encoding for apolipoprotein E, increases the chances of developing Alzheimer disease three times, whilst inheriting both copies increases one's risk eightfold.[3]
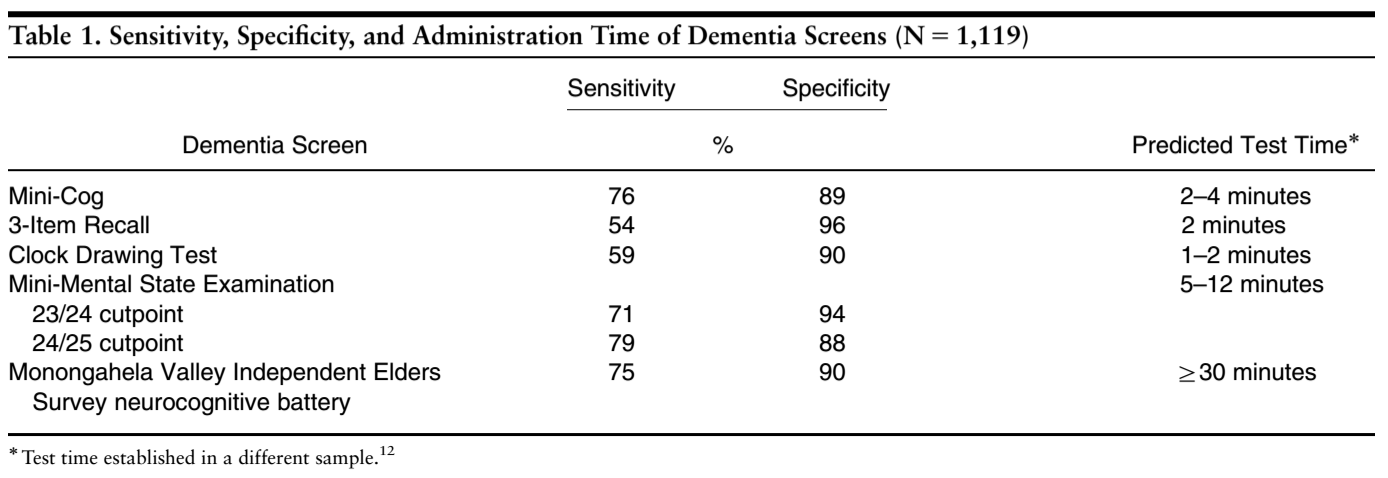
Screening Tools[edit | edit source]
Objective tools have been validated in the practice of physical therapy in order to screen for AD such as the Mini-Cog, Mini-Mental State Exam (MMSE), Clock-Drawing, & Neurobehavioral Cognitive Status Exam. A pilot study developed a study protocol aimed at aiding the early detection of dementia disorders using the Timed Up-and-Go (TUG) test with the verbal task of naming different animals[16].
Screening tools can be chosen based upon sensitivity, specificity and time to administer the screen.
Mini-Mental State Exam was validated for detecting possible dementia, however, time to administer the exam keeps physicians from using it. The MMSE takes 5-12 minutes to administer and is composed of 20 questions in 5 categories to observe orientation, memory, attention-concentration, language and constructing[17].
- Cut off scores: (out of 25)
- ≥ 24 = no impairment
- 18-23 = mild impairment
- ≤ 17 = severe impairment
- < 23 is generally accepted as indicating cognitive impairment and was associated with the diagnosis of dementia in at least 79% of cases (Lancu & Olmer, 2006) [18]
Mini-Cog takes 2-4 minutes to administer and combines constructing (clock drawing) and memory. [19]
- Below are current findings for ruling the differential diagnosis of AD in or out, due to how the tests perform in terms of sensitivity, it would be best to cluster these tests in order to rule in the possibility of dementia or AD.
- A score < 3 indicates clinically meaningful cognitive impairment in a score out of 10
Causes[edit | edit source]
The definitive cause of Alzheimer’s disease is still unknown. It is believed that early-onset Alzheimer’s is caused by a genetic mutation. Late-onset Alzheimer’s disease is caused by complex changes that occur in the brain over a period of time. It is believed that a combination of factors from the environment, genetic, and lifestyle. The importance of a single factor may play on an individual is different among everyone with the disease. Refer to risk factors under Characteristics/ Clinical Presentation above for further information. [21]
Systemic Involvement[edit | edit source]
The most noticeable symptoms initially are the cognitive and memory-related symptoms. However, Alzheimer's disease can affect other parts of the body causing symptoms other than those affecting memory and cognition. Often abnormal motor signs can be apparent depending on the area of the brain affected by the disease. The presence of tremors can be associated with increased risk for cognitive decline, the presence of bradykinesia with increased risk for functional decline, and the presence of postural-gait impairments with increased risk of institutionalization and death. Additionally, patients may develop disorders of sleeping, eating, and sexual behavior.[22]
Medical Management[edit | edit source]
There is currently no cure for Alzheimer's Disease, so medical management is focused on maintaining the quality of life, maximizing function, enhancing cognition, fostering a safe environment and promoting self engagement[23]. Maximizing dementia functioning involves monitoring the patient's health and cognition, patient and family education, initiation of pharmacological and non-pharmacological treatments.
- Cognitive symptom treatment
- Although the disease progression cannot be altered, it may be slowed by the pharmacological medication listed above
- Behavioral and psychological symptom treatment
- Agitation, aggression, depression, and psychosis are the primary cause of assisted living or nursing home placement.
- Assessment of behaviors occurring suddenly is important to increase patient comfort, security, and ease of mind.
- Monitoring Alzheimer’s disease
- Patients should return on a regular basis in order for the physician to monitor the course of Alzheimer’s disease (behavioral and cognitive changes).
- Regular follow-up appointments allow for the adaptation of treatment styles to fit the needs of the patient.
- Non medical/social Issues the patients need to address:
- Need for ongoing support & information
- A living will or power of attorney
- Review of finances/planning for future and end of life care
- Alternative Treatment
- There are concerns regarding alternative treatments in addition to physician-prescribed medicine. If any concerns are questions brought to attention, the physician should be notified.
- Importance of Caregiver
- Many caregivers seek to meet the needs of the physician and the patient which increases rates of stress and depression. Physicians should continue to monitor the status of the caregivers watching out for burnout and providing them with resources as well.
- Aducanumab
- This is a amyloid beta-directed monoclonal antibody drug that is intended to treat Alzheimer's disease. It is sold under the brand name Aduhelm and is intended to target segregated forms of amyloid beta (Aβ) in the brains of people with Alzheimer's disease to lower its buildup.[24]
- The drug was approved by the Food and Drug Administration in June 2021 under the accelerated approval pathway. The FDA reported that the drug is a "first-of-its-kind-treatment" approved for Alzheimer's disease and is the first new treatment for the disease since 2003. Aducanumab was also reported to be the first drug that addresses the pathophysiology of Alzheimer's disease. [24]
Physical Therapy Management[edit | edit source]
It is important to modify risk factors that can be changed through lifestyle activities. Hypertension has been shown to interact with a particular genotype that is at risk for developing Alzheimer’s disease. This interaction increases amyloid deposition in cognitively healthy middle-aged and older adults. Thus, when at-risk it is important to manage blood pressure, which can be done through exercise[26].
Physical activity is important to incorporate in a patient’s with Alzheimer’s disease life, and the sooner the better. “Earlier application of physical activity to mitigate pathological processes and to assuage cognitive decline is imperative given recent evidence from clinical trials suggesting that interventions applied earlier in the course of Alzheimer's Disease are more likely to achieve disease modification, whereas those applied later have a significant but more limited effect after the emergence of neuronal degeneration.”[27]
A community-based exercise program has been shown to improve multiple domains of life for individuals with Alzheimer's. In a study by Vreugdenhil et al., community-dwelling individuals with Alzheimer's added a daily home exercise program and walking under supervision to a usual treatment plan. Those participating in the additional exercise improved cognition, mobility, and instrumental activities of daily living[28].
It has been suggested that aerobic exercise in the form of walking and upper limb cycle ergometer, in particular, helps to improve exercise tolerance as well as quality of life in individuals with Alzheimer's[29]. Strength training in addition to aerobic training has been supported in the research. The combination of both activities have shown greater improvements in cognition than aerobic training alone[30].
Individuals with dementia are at an increased risk for falling compared to the average population of community-dwelling older adults. [31] A research study suggests that poor visual acuity resulted in poorer executive function, which further caused more inadequate balance control, thus demonstrating the importance of assessing executive functions besides vision and balance in older individuals living with Alzheimer's dementia.[32]
Preliminary research has been conducted looking at falls prevention training for individuals with intellectual disorders such as Alzheimer’s disease. A study found that using a modified Otago Exercise program was effective at decreasing falls risk for some adults with intellectual disabilities[33]. A pilot study found that the Berg Balance Scale had relative reliability values that support its use in clinical settings. However, MDC values are not established for this population[34]. More research is needed in this area to best assess falls risk in this population.
Frequently, when a physical therapist works with a patient who has been diagnosed with Alzheimer's Disease, the patient may be in a structured living environment because they have progressed to a stage in the disease where their caregivers can not give the patient the proper attention that they need. Physical therapy can provide the patient with an activity that the patient can perform successfully at and it also can help to improve their breathing, mobility, and endurance. Restlessness and wandering can be typical of patients with Alzheimer's patients and may be managed with physical therapy (by releasing some of the energy through exercises). These exercises can help to reduce the night time wanderings called sundowning.
Transition Care[35] provides time-limited, goal-oriented and therapy-focused packages of services to older people after a hospital which includes low-intensity therapy—such as physiotherapy and occupational therapy—social work and nursing support or personal care. It is designed to improve independence and functioning in order to delay their entry into residential aged care. A qualitative research study suggests better outcomes in older patients (above 80yrs) with family participation to assist physiotherapy care in a Transition care setting[36].
Group therapy is also successful with patients with Alzheimer's disease, but the session must not provide more stimulation than the patient is able to tolerate. Repetition and encouragement are also very important to help keep the patient's confidence high and to help with remembering the exercises. Knowing the patient is important to the therapist because it can allow for better communication, by using words and terms that the specific patient may be more familiar with. The Preferred Practice Pattern is 5E: Impaired Motor Function and Sensory Integrity Associated with Progressive Disorders of the Central Nervous System. The physical therapist can use the Global Deterioration Scale to assess the level of dementia. When a patient with Alzheimer's is placed in a comprehensive cognitive stimulation program it enhances the neuroplasticity of the patient. The exercise can also help to improve mobility, balance, and ROM for the patient as well as improve the mood[37].
Staying physically and socially active can possibly help to decrease the risk of dementia along with staying mentally active. A randomized controlled trial showed favorable outcomes with exercise and horticultural intervention programs for older adults with depression and memory problems[38].
Dietary Management[edit | edit source]
It has been found that maintaining a healthy diet may help to prevent or slow the progression of Alzheimer's. It is suggested that the diet be low in fat, high in omega-3 oils, and high in dark vegetables and fruits, also adding vitamin C to the diet along with coenzyme Q10, and folate may work to lower the risk of Alzheimer's. There does not seem to be one single aspect of diet that provides neuroprotection, rather than the items work together to decrease the risk of AD.[39]There is also some interest in the use of antioxidants such as vitamin E and ginkgo, along with anti-inflammatory agents, and estrogen replacement therapy for women.[40]
Differential Diagnosis[edit | edit source]
- Pick's Disease
- Lewy Body Dementia
- Frontotemporal Dementia
- Dementia from multiple medications
- Other potentially reversible causes of dementia
Low Resource Health Settings[edit | edit source]
More than half of all people with dementia are from low and middle-income countries. Alzheimer’s disease, other dementias, and non-communicable diseases are expected to continue to be a burden on health systems throughout sub-Saharan Africa, as country populations age and communicable disease mortality and morbidity go down [41]. The number of people with Alzheimer's disease and dementia in general is estimated to increase far more rapidly in the upper middle, lower middle and low-income countries (LMICs) than in the high-income countries [42]. There is a general lack of awareness of the disease among the population, therefore patients don't seek for medical care and do not get the treatment they need. Hence, it is under-recognized, underdisclosed, undertreated, and undermanaged, particularly in LMICs[43]. The living environment also often poses little cognitive challenge because families may not understand their relative’s behavior [44]. Many of the cognitive and functional assessment tools used in LMICs were originally developed and validated in High Income Countries. There is a need to adapt it to be used more effectively in LMICs [45].
References[edit | edit source]
- ↑ Anand, R., Gill, K.D. and Mahdi, A.A. (2014) 'Therapeutics of Alzheimers disease: past, present and future', Neuropharmacology, 76, 27-50
- ↑ 2.0 2.1 Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Radiopedia Alzheimer disease Available:https://radiopaedia.org/articles/alzheimer-disease-1?lang=gb (accessed 16.1.2023)
- ↑ Kumar A, Sidhu J, Goyal A, Tsao JW. Alzheimer disease. Available:https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed 16.1.2023)
- ↑ Alzheimer's & Dementia Testing Advances | Research Center [Internet]. Alzheimer's Association. [cited 2017Apr2]. Available from: http://www.alz.org/research/science/earlier_alzheimers_diagnosis.asp
- ↑ Alzheimer's and Dementia Causes, Risk Factors | Research Center [Internet]. Alzheimer's Association. [cited 2017Apr1]. Available from: http://www.alz.org/research/science/alzheimers_disease_causes.asp#apoe
- ↑ Alzheimer's and Dementia Causes, Risk Factors | Research Center [Internet]. Alzheimer's Association. [cited 2017Apr1]. Available from: http://www.alz.org/research/science/alzheimers_disease_causes.asp#apoe
- ↑ Latest Alzheimer's Facts and Figures [Internet]. Latest Facts; Figures Report | Alzheimer's Association. 2016 [cited 2017Apr1]. Available from: http://www.alz.org/facts/
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Porth C. Pathopysiology Concepts of Altered Health States. Philadelphia PA: Lippincott and Wilkins; 2005.
- ↑ Stages of Alzheimer's Symptoms [Internet]. Alzheimer's Association. [cited 2017Apr1]. Available from: http://www.alz.org/alzheimers_disease_stages_of_alzheimers.asp
- ↑ Porth C. Pathopysiology Concepts of Altered Health States. Philadelphia PA: Lippincott and Wilkins; 2005.
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Alzheimer's & Dementia Testing Advances | Research Center [Internet]. Alzheimer's Association. [cited 2017Apr3]. Available from: http://www.alz.org/research/science/earlier_alzheimers_diagnosis.asp
- ↑ Cedervall Y, Stenberg AM, Åhman HB, Giedraitis V, Tinmark F, Berglund L, Halvorsen K, Ingelsson M, Rosendahl E, Åberg AC. Timed Up-and-Go Dual-Task Testing in the Assessment of Cognitive Function: A Mixed Methods Observational Study for Development of the UDDGait Protocol. International journal of environmental research and public health. 2020 Jan;17(5):1715.
- ↑ Benson AD, Slavin MJ, Tran T-T, Petrella JR. Screening for Early Alzheimer's Disease: Is There Still a Role for the Mini-Mental State Examination? The Primary Care Companion [Internet]. [cited 2017Apr1]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1079697/
- ↑ Rehab Measures - Mini-Mental State Examination [Internet]. The Rehabilitation Measures Database. [cited 2017Apr1]. Available from: http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=912
- ↑ Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a Screen for Dementia: Validation in a Population-Based Sample. Journal of the American Geriatrics Society. 2003;51(10):1451–4.
- ↑ Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a Screen for Dementia: Validation in a Population-Based Sample. Journal of the American Geriatrics Society. 2003;51(10):1451–4.
- ↑ About Alzheimer's Disease: Causes [Internet]. National Institutes of Health. U.S. Department of Health and Human Services; [cited 2017Apr3]. Available from: https://www.nia.nih.gov/alzheimers/topics/causes
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Medical Management and Patient Care [Internet]. Alzheimer's Association. [cited 2017Apr1]. Available from: http://www.alz.org/health-care-professionals/medical-management-patient-care.asp
- ↑ 24.0 24.1 "FDA Grants Accelerated Approval for Alzheimer's Drug". U.S. Food and Drug Administration (FDA) (Press release). 7 June 2021. Accessed on 29 January 2022. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- ↑ Pollom E, Little J. PT Management of Alzheimer's Disease [Internet]. YouTube. YouTube; 2017 [cited 2017Apr2]. Available from: https://www.youtube.com/watch?v=rW3rQ73rQFE&t=8s
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Phillips, C. et al. "The Link Between Physical Activity And Cognitive Dysfunction In Alzheimer Disease". Physical Therapy 95.7 (2015): 1046-1060. Web. 1 Apr. 2017.
- ↑ Vreugdenhil, Anthea et al. "A Community-Based Exercise Programme To Improve Functional Ability In People With Alzheimer’S Disease: A Randomized Controlled Trial". Scandinavian Journal of Caring Sciences 26.1 (2011): 12-19. Web. 1 Apr. 2017.
- ↑ Mahmoud S. “Role of aerobic exercise training in changing exercise tolerance and quality of life in Alzheimer's disease”. European journal of general medicine. 2011;8(1):1-6. Web. 1 Apr. 2017.
- ↑ Manckoundia, Patrick et al. "Impact Of Ambulatory Physiotherapy On Motor Abilities Of Elderly Subjects With Alzheimer's Disease". Geriatrics;; Gerontology International 14.1 (2013): 167-175. Web. 1 Apr. 2017.
- ↑ Renfro M, Bainbridge D, Smith M. Validation of Evidence-Based Fall Prevention Programs for Adults with Intellectual and/or Developmental Disorders: A Modified Otago Exercise Program. Frontiers in Public Health. 2016;4. Web. 1 Apr. 2017.
- ↑ Hunter SW, Divine A, Madou E, Omana H, Hill KD, Johnson AM, Holmes JD, Wittich W. Executive function as a mediating factor between visual acuity and postural stability in cognitively healthy adults and adults with Alzheimer’s dementia. Archives of Gerontology and Geriatrics. 2020 Apr 19:104078.
- ↑ Muir-Hunter S, Graham L, Montero Odasso M. Reliability of the Berg Balance Scale as a Clinical Measure of Balance in Community-Dwelling Older Adults with Mild to Moderate Alzheimer Disease: A Pilot Study. Physiotherapy Canada. 2015;67(3):255-262. Web. 1 Apr. 2017.
- ↑ Renfro M, Bainbridge D, Smith M. Validation of Evidence-Based Fall Prevention Programs for Adults with Intellectual and/or Developmental Disorders: A Modified Otago Exercise Program. Frontiers in Public Health. 2016;4. Web. 1 Apr. 2017.
- ↑ Transition Care Programme. Aging and aged care.Accessed from ☀https://agedcare.health.gov.au/programs-services/flexible-care/transition-care-programme on 4/12/2019.
- ↑ Lawler K, Taylor NF, Shields N. Family-assisted therapy empowered families of older people transitioning from hospital to the community: a qualitative study. Journal of physiotherapy. 2019 Jun 13.
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Makizako H, Tsutsumimoto K, Makino K, Nakakubo S, Liu-Ambrose T, Shimada H. Exercise and Horticultural Programs for Older Adults with Depressive Symptoms and Memory Problems: A Randomized Controlled Trial. Journal of Clinical Medicine. 2020 Jan;9(1):99.
- ↑ Goodman CC, Fuller KS. Pathology: implications for the physical therapist. St. Louis, MO: Elsevier Saunders; 2015.
- ↑ Porth C. Pathopysiology Concepts of Altered Health States. Philadelphia PA: Lippincott and Wilkins; 2005.
- ↑ Mubangizi V, Maling S, Obua C, Tsai AC. Prevalence and correlates of Alzheimer’s disease and related dementias in rural Uganda: cross-sectional, population-based study. BMC geriatrics. 2020 Dec;20(1):1-7.
- ↑ Global Prevalence. Available from: https://www.dementiastatistics.org/statistics/global-prevalence/ ( Accessed, 20/09/2021).
- ↑ Ferri CP, Jacob KS. Dementia in low-income and middle-income countries: different realities mandate tailored solutions. PLoS medicine. 2017 Mar 28;14(3):e1002271.
- ↑ George-Carey R, Adeloye D, Chan KY, Paul A, Kolčić I, Campbell H, Rudan I. An estimate of the prevalence of dementia in Africa: a systematic analysis. Journal of global health. 2012 Dec;2(2).
- ↑ Sexton C, Snyder HM, Chandrasekaran L, Worley S, Carrillo MC. Expanding Representation of Low and Middle Income Countries in Global Dementia Research: Commentary From the Alzheimer's Association. Frontiers in Neurology. 2021 Mar 15;12:271.
</div>