Physical Activity as an Anti-Aging Medicine
Original Editor - Tolulope Adeniji
Introduction[edit | edit source]
Chronic illnesses are commonly found among elderly individuals due to the effects of aging on the body's systems. Older adults often experience conditions such as neurocognitive disorders, osteoarthritis, and cardiovascular diseases, which can hinder their activity levels and make them more susceptible to sedentary-related ailments like obesity. Lately, there has been an increasing focus on the importance of physical activity and exercise in enhancing functionality, addressing impairments, and mitigating the impact of aging on this particular population.
The purpose of this study is to explore various physical activities and exercises that have the potential to reverse or alleviate the aging process.
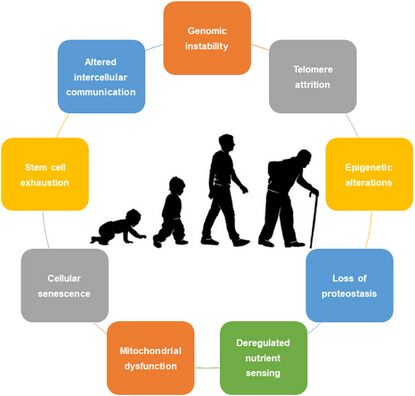
Hallmarks of Ageing[edit | edit source]
Aging is characterized by a complex and intra-individual process.
- Evidence has recently shown that nine cellular and molecular characteristics could explain the aging process, including genomic instability; telomeric attrition; epigenetic alterations; loss of proteostasis; and deregulated nutrient sensing,
- Additional factors that contribute to this phenomenon are impaired mitochondrial function, cellular aging, depletion of stem cells, and disrupted communication between cells[1].
Physical activity[edit | edit source]
Physical activity deals with skeletal movement that results in energy expenditure
- Exercise is a subset of physical activity that is planned, structured and repetitive with the aim of maintaining or improving physical fitness[2].
- Physical fitness is a health or skill attribute that can be quantified with objective measures.[2].
Physical activity and Hallmarks of Ageing[edit | edit source]
America College of Sport Medicine[3] had shown that physical activity among older adults has multiple benefit including:
- Lower rates of all-cause mortality eg reducing risk of coronary artery disease, high blood pressure, stroke, type 2 diabetes, colon cancer and breast cancer.
- Improves chronic conditions such as type 2 diabetes , cardiovascular diseases and bone density enhancement,
Physical Activity and Genomic Instability[edit | edit source]
Zhang et al. noted that more than 30 minutes of physical activity has a potential positive effect on global genomic DNA methylation. DNA methylation often inhibits the expression of certain genes. For example, the methylation process might stop a tumor-causing gene from “turning on,” preventing cancer[4].[5] and Dimauro et al.,[6] also supported these findings as the authors reported that physical activity enhances genomic stability among patients with diabetes. Genome stability is crucial to accurately transmit all genetic information to progeny (or daughter cells upon cell division) to protect genetic integrity and prevent disease. Unfortunately all of us will (personally, or in the people surrounding us) experience the fact that this process is not without error, as in eg cancer[7].
Physical Activity and Telomere Attrition[edit | edit source]
Physical activity of moderate intensity exercise has shown to preserve telomere length[8] [9]and even sometimes exercise has potential to restoring defective telomere length[8]A new systematic review and meta-analysis of randomized controlled trials suggests that exercise positively affects the telomere length compared to usual care or inactivity and aerobic exercise performed at moderate intensity over six months or longer is the most effective in slowing the rate of telomere shortening. [10]
Physical Activity and Epigenetic Alterations[edit | edit source]
The epigenome encompasses the chemical alterations that occur to DNA and histone proteins within a cell. These alterations involve functional modifications of the genome, which are driven by processes such as DNA methylation, histone modification, and microRNA expression.[11].
Physical activity of different intensity exercise especially moderate level had shown to positively alter the epigenome[12]. This was demonstrated in the biomakers of epigenome measured. This include Hypermethylation in one CpG site of AMPKA2 and Upregulation of miR-1 and miR-133a etc. This further improves skeletal muscle damage, cardiac stress, necrosis, and systemic inflammation[12].
Physical Activity and Loss of Proteostasis[edit | edit source]
One characteristics of ageing has been associated with impaired protein homeostasis, also known as proteostasis. Proteostasis includes autophagy and proteasomal degradation etc.[13] Campos et al.,[14] had shown that a 4 weeks of aerobic exercise training on treadmill for 5 treatment session in a week for an hour per day prior to primary disease of nervous system (neurogenic myopathy) improves skeletal muscle autophagic flux and proteostasis[14].
Physical Activity and Deregulated Nutrient sensing[edit | edit source]
Being able to sense and respond to fluctuations in environmental nutrient levels is a necessary for life. Lack of nutrients is a selective pressure that has shaped the evolution of most cellular processes. Various pathways exist to detect levels of sugars, amino acids, lipids, and surrogate metabolites within cells and outside of cells. These pathways are integrated and coordinated at the organismal level through hormonal signals. In human metabolic diseases, nutrient-sensing pathways are frequently disrupted.[15]. Exercise plays an important in our nutrient-sensing systems, promoting a beneficial anabolic cellular state. [1]
Physical Activity and Mitochondrial dysfunction[edit | edit source]
Cytochrome c-oxidase deficiency in skeletal muscle fibers resulting in higher levels of mitochondrial DNA (mtDNA) mutations is common in older adults. However, resistance exercises improve this deficiency and it has even been shown that a 6-month resistance training program reversed the ageing of transcriptional signatures, especially in younger adults.[1]
Physical Activity and Cellular Senescence[edit | edit source]
Cellular senescence is characterized by an irreversible arrest of the cell cycle. This is caused by a multi-factorial mechanism that includes telomere shortening, other forms of genotoxic stress, or mitogens or inflammatory cytokines. Particularly, moderate aerobic exercise has been shown to improve cellular senescence.[1]
Physical Activity and Stem Cell Exhaustion[edit | edit source]
Endothelium reparative capacity of endothelial progenitor cells is essential for tissue proliferation and this decline as a result of ageing, however, regular physical activities has been shown to have potential not only to improve this cells but in attenuating stem cell exhaustion[1].
Physical Activity and Altered Intercellular Communication[edit | edit source]
Engaging in physical activity, particularly moderate aerobic exercises, triggers the release of specific chemicals during muscle contraction, thereby enhancing the communication between cells that have undergone changes. Some of these chemicals are myokine secretion (proteins, growth factors, cytokines, or metallopeptidases) and IL-6, which induces the production of anti-inflammatory cytokines that further improve or protect intercellular communication[1].
Conclusion[edit | edit source]
Physical activity when graded is essential medication for delaying or reversing ageing or, better still, for alleviating conditions associated with the aging process.
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Rebelo-Marques A, De Sousa Lages A, Andrade R, Ribeiro CF, Mota-Pinto A, Carrilho F, Espregueira-Mendes J. Aging hallmarks: the benefits of physical exercise. Frontiers in endocrinology. 2018 May 25;9:258.
- ↑ 2.0 2.1 Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public health rep. 1985 Mar 1;100(2):126-31.
- ↑ Chodzko-Zajko WJ, Proctor DN, Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. Exercise and physical activity for older adults. Medicine & science in sports & exercise. 2009 Jul 1;41(7):1510-30.
- ↑ Healthline DNA Methylation: Can Your Diet Reduce Your Risk of Disease? Available:https://www.healthline.com/health/methylation (accessed 25.7.2022)
- ↑ Zhang FF, Cardarelli R, Carroll J, Zhang S, Fulda KG, Gonzalez K, Vishwanatha JK, Morabia A, Santella RM. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011 Mar 1;6(3):293-9.
- ↑ Dimauro I, Sgura A, Pittaluga M, Magi F, Fantini C, Mancinelli R, Sgadari A, Fulle S, Caporossi D. Regular exercise participation improves genomic stability in diabetic patients: an exploratory study to analyse telomere length and DNA damage. Scientific reports. 2017 Jun 23;7(1):1-2.
- ↑ LUMC Genome stability Available from:https://www.lumc.nl/org/humane-genetica/research/research-line-2/Genomestability/ (accessed 25.7.2022)
- ↑ 8.0 8.1 Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget. 2017 Jul 4;8(27):45008.
- ↑ Tucker LA. Physical activity and telomere length in US men and women: An NHANES investigation. Preventive medicine. 2017 Jul 1;100:145-51.
- ↑ Song S, Lee E, Kim H. Does Exercise Affect Telomere Length? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina. 2022 Feb 5;58(2):242.
- ↑ Grazioli E, Dimauro I, Mercatelli N, Wang G, Pitsiladis Y, Di Luigi L, Caporossi D. Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC genomics. 2017 Nov;18(8):111-23.
- ↑ 12.0 12.1 Barrón-Cabrera E, Ramos-Lopez O, González-Becerra K, Riezu-Boj JI, Milagro FI, Martínez-López E, Martínez JA. Epigenetic Modifications as Outcomes of Exercise Interventions Related to Specific Metabolic Alterations: A Systematic Review. Lifestyle genomics. 2019;12(1-6):25-44.
- ↑ Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Morán M, Emanuele E, Joyner MJ, Lucia A. Exercise attenuates the major hallmarks of aging. Rejuvenation research. 2015 Feb 1;18(1):57-89.
- ↑ 14.0 14.1 Campos JC, Baehr LM, Gomes KM, Bechara LR, Voltarelli VA, Bozi LH, Ribeiro MA, Ferreira ND, Moreira JB, Brum PC, Bodine SC. Exercise prevents impaired autophagy and proteostasis in a model of neurogenic myopathy. Scientific reports. 2018 Aug 7;8(1):1-4.
- ↑ Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015 Jan;517(7534):302-10. Available from:https://pubmed.ncbi.nlm.nih.gov/25592535/ (accessed 25.7.2022)