CREST Syndrome
Original Editor - Aya Alhindi
Top Contributors - Aya Alhindi, Kim Jackson and Khloud Shreif
Description[edit | edit source]
CREST syndrome (also known as Cutaneous systemic sclerosis or limited scleroderma ) is an autoimmune disease that has been defined as a subtype of progressive systemic sclerosis (SSc) with limited skin involvement. [1]The word "CREST " is an acronym for the clinical features that are seen in a patient with this disease:
- Calcinosis- when calcium salts are deposited into the skin and subcutaneous tissue.[2]
- Raynaud's phenomenon.
- Esophageal dysmotility-which can cause difficulty in swallowing.
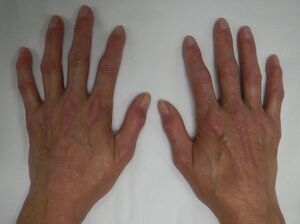
- Sclerodactyly- scleroderma in which the fingers become thin and shiny with sclerotic skin at the tip due to subcutaneous and intracutaneous calcinosis and diffused fibrosis of the collagen.[3]
- Telangiectasia-small widened blood vessels on the skin.[4]
Epidemiology[edit | edit source]
Wide variation in prevalence of systemic sclerosis was observed, with slightly higher estimates reported in North America (13.5–44.3 per 100,000 individuals) compared to Europe (7.2–33.9 per 100,000 individuals), which may be a true reflection of epidemiological variation or an artifact of clinical data analyses.[5] The apparent increase in both incidence and prevalence over the last 50 years is most likely due to improved classification, earlier diagnosis, and survival. CREST syndrome may account for 22-25% of all occurrences of systemic sclerosis, according to serum antibody investigations; however, epidemiologic research specifically looking at CREST syndrome are missing.[6] SSc diagnosis was reported to occur at the ages of 33.5-59.8 years in Europe and 46.1-49.1 years in North America, and to occur more commonly in women (female:male ratio of 3.8-11.5:1 in Europe and 4.6-15:1 in North America). Women have continuously greater prevalence and incidence rates, indicating a clinically significant difference in the occurrence of SSc across genders.[5]
Pathophysiology[edit | edit source]
The pathogenesis of SSc involves a classical triad of key mechanisms:[7]
- Endothelial dysfunction and apoptosis of endothelial cells.
- Uncontrolled activation of adaptive and innate immunity (notably including M1 inflammatory and M2 pro-fibrotic macrophages).
- Over-production of extracellular matrix (ECM) components by chronically activated myofibroblasts, resulting in the formation of a stiff and fibrotic extracellular matrix in numerous organs, interfering with their function.
Myofibroblasts are the primary contributors to ECM formation and fibrosis.In SSc, myofibroblasts are derived from a range of tissue-resident mesenchymal progenitor cell types, such as fibroblasts, pericytes, microvascular endothelial cells, and vascular preadipocytes]. In SSc, myofibroblasts undergo substantial epigenetic remodelling as well as metabolic changes such as increased glycolysis and altered NAD+ homeostasis.Furthermore, myofibroblasts in SSc exhibit apoptosis resistance as well as unregulated production of extracellular matrix (ECM) components such as collagens, tenascin C, and fibronectin. In turn, these released extracellular components can activate myofibroblasts either directly via innate immunological sensors such as TLR-4, or indirectly by mechano-sensing of increased matrix stiffness by integrins in an FAK dependent way. [7]
Regarding limited scleroderma, although the primary cause is unknown, it is reasonable to speculate that vascular endothelial cell abnormalities induce mononuclear infiltration, and that the resulting changes in TH1 and/or TH2 cell and cytokine balance result in abnormal fibroblast activity and increased collagen deposition.[8]
Diagnosis[edit | edit source]
In the absence of a diagnostic test proving the absence or presence of SSc, the diagnosis is based on a combination of clinical and laboratory findings. According to the latest classification scheme from 2013, SSc is confirmed by:[9]
Major Criteria:
- skin thickening of the fingers of both hands extending proximal to the metacarpophalangeal joints (MCP) is sufficient to classify a subject as having SSc.
- skin thickening sparing the fingers’ are classified as not having SSc.
Minor criteria:
- Skin thickening of the fingers -Puffy fingers and Sclerodactyly of the fingers (distal to the metacarpophalangeal joints but proximal to the proximal interphalangeal joints)
- Fingertip lesions -Digital tip ulcers and Fingertip pitting scars
- Telangiectasia
- Abnormal nailfold capillaries
- Pulmonary arterial hypertension and/or interstitial lung disease
- Raynaud’s phenomenon
- SSc-related autoantibodies:
- Anticentromere.
- Anti–topoisomerase I [anti–Scl-70].
- Anti–RNA polymerase III.
Etiology[edit | edit source]
While the exact cause of CREST syndrome is unknown, it is thought to be the result of a complex interplay of genetic, immunological, and environmental variables.
Calcinosis[edit | edit source]
Approximately 40% of patients with limited cutaneous SSc complicating from calcinosis (or dystrophic calcification) which is the accumulation of insoluble calcified material in the soft tissues, occurring in the presence of normal calcium and phosphate metabolism. The cause of how or why these crystals form in patients with SSc is not well understood.There is some poorly understood factors have all been proposed to contribute to calcinosis including:[10]
Chronic hypoxia
characterised by:
- Digital ulcers
- Loss of digital tip
- Abnormal capillary drop-outs seen by nailfold Capillaroscopy
Repetitive trauma
- Based on common locations of these deposits such as the fingertips and extensor surfaces of extremities.
Localised structural damage
Raynaud's phenomenon[edit | edit source]
Raynaud’s phenomenon secondary to SSc occurs in 90% of patients and is often the earliest clinical manifestation to occur.[11]It is vasospastic disorder that characterized by frequent and sudden drops in blood flow to the fingertips, often in response to cold temperatures. Raynaud phenomenon is a symptom complex caused by impaired digital perfusion and can occur as a primary phenomenon or secondary to a wide range of underlying causes. [12]
The etiology of SSc-associated Raynaud phenomenon includes factors such as endothelial cell injury (possibly autoantibody mediated); an imbalance between vasoconstrictor and vasodilator molecules (such as endothelin 1 and nitric oxide, respectively); structural microvascular changes from progressive microangiopathy; and intravascular events that lead to luminal occlusive disease. [12]
Esophageal dysmotility[edit | edit source]
SSc and gastrointestinal manifestations have been proven to be associated , with more than 90% of SSc patients also having various types of gastrointestinal dysfunction, which is considered the third greatest cause of death in SSc patients . Esophageal micro-reflux, manifested as dysphagia and reflux heartburn, is the most common indication of gastrointestinal dysfunction involvement and, to some extent, exacerbates the existing interstitial lung diseases. Furthermore, Barrett's oesophagus, esophageal stenosis, and esophageal cancer induced by esophageal motility problems may worsen the prognosis of SSc patients.[13]The etiology of esophageal motility disorders in patients with SSc is still uncertain.Many functional tests show the presence of vascular damage, fibrosis, and inflammatory illness, however these three factors may not be a complete picture and may lead to undetected etiology. Previously, some studies reporting the related pathological observations revealed esophageal muscle atrophy without evidence of vascular damage, fibrosis, or inflammatory infiltration. However, the exact mechanism of esophageal muscle atrophy is not known. [13]
Sclerodactyly[edit | edit source]
Sclerodactyly develops from a perivascular inflammatory infiltration in the dermis.[14]Although the cause of this inflammatory process is still unknown,it is believed that mucopolysaccharide, glycoprotein, and collagen (types I and III) deposition in the dermis causes the edematous phase of skin involvement.As collagen deposition progresses, the dermis becomes sclerotic rather than edematous. Meanwhile, in small arteries, a similar mechanism happens and in the intima, mucinous deposition occurs. [15]The adventitia is initially invaded by inflammatory cells before becoming fibrotic. This process causes artery narrowing, followed by arterial collapse or thrombosis.As a result the tissue becomes ischemic.Fibrosis typically disappears years after the start of skin changes, leaving atrophic skin behind.[15]
Telangiectasia[edit | edit source]
Prominent and numerous telangiectasia are a common clinical symptoms of scleroderma.The etiology of telangiectasia in general, and CREST in particular, is unknown. Models have not explained the preference for hands, face, and mucosa, as well as their proclivity to enlarge in diameter with time. Venous hypertension as a cause appears implausible given the rarity of high venous pressures in the foot. While both Raynaud's phenomenon and telangiectasia are present in CREST, telangiectasia is not present in primary Raynaud's disease, implying that telangiectasia is not caused by recurrent vasospasm or vasoconstriction. Telangiectasia is also widespread on the face, which is unaffected by Raynaud's phenomenon.[16]
Medical Management[edit | edit source]
Because a scleroderma diagnosis will affect a patient's physical and psychological well-being, a comprehensive approach to care should be taken. An assessment of organ involvement is required, as well as patient education about the clinical course, patient and family support, and treatment based on disease severity and organ involvement. A rheumatologist should be consulted.[17]
Organ-specific treatment can have a direct effect on outcomes such as mortality, disease progression, and quality of life issues.[18]
| Problem | Treatment |
|---|---|
| Arthralgias | NSAIDs, methotrexate, Cox-2 inhibitors |
| End-stage lung disease | Lung transplantation |
| Esophageal reflux | Proton pump inhibitors, metoclopramide |
| Intestinal dysmotility | Antibiotics, if malabsorption present; prokinetics |
| Pulmonary hypertension | Calcium channel blockers, epoprostenol |
| Lung inflammation | Cyclophosphamide |
| Inflammatory myositis | Methotrexate, prednisone |
| Raynaud’s phenomenon | Calcium channel blockers, cold avoidance,
angiotensin receptor blockers, nitroglycerin, digital sympathectomy |
| Renal crisis | Aggressive blood pressure control, including
angiotensin 1- converting enzyme inhibitors; dialysis |
Raynaud’s phenomenon management[edit | edit source]
In approximately 50% of the patients Raynaud’s phenomenon is often very severe and can progresses to digital ulceration (DU).[19]First, Raynaud's phenomenon is treated, and then digital ulceration is treated. The two are purposely combined since optimising Raynaud's phenomenon treatment is a critical initial step in the prevention and treatment of SSc-related digital ulcers. Lifestyle changes (including patient education) and vasoactive medication treatments are recommended for the best management of SSc-related Raynaud's phenomenon. [20]
Pharmacological management of digital ulceration (DU) can include:[20]
- Vasoactive therapies.
- Other pharmacological therapies (excluding procedural treatments) including antibiotic therapy and analgesia.
- Procedural pharmacological therapies.
- Treatment of the acute DU (this can be a medical emergency).
Calcinosis management[edit | edit source]
Calcinosis cutis is difficult to treat pharmacologically, and a range of medications, including bisphosphonates, intralesional corticosteroids, aluminium hydroxide, warfarin, and diltiazem, have been attempted with poor effectiveness. The current available therapeutic option is local excision of uncomfortable or ulcerated nodules, but local recurrence is prevalent. [21]
Esophageal dysmotility management[edit | edit source]
In those with systemic/localized scleroderma (SSc) or limited scleroderma, the gastrointestinal tract (GI) is the second most affected organ system. SSc can impact any portion of the GI tract, from the mouth cavity to the anorectum. [22]
| GI part | Treatment |
|---|---|
| Oral cavity | rehabilitation via orofacial exercises and the administration of cevimeline, pilocarpine, muscarinic agonists, and artificial saliva. |
| Esophagus | Lifestyle management including head elevation at night, excluding triggering foods/substance abuse, and consuming small/frequent meals during the day.
Proton pump inhibitors (PPIs). Endoscopic dilatationEndoscopic ablation or resection of dysplastic epithelium using photochemical, thermal, or radio ablation energy is recommended in Barrett’s esophagus. |
| Stomach | Dietary modifications (low-fat/fiber-based diet and vitamin supplementation) are the first line for gastroparesis |
| Small intestine | Antibiotics such as ciprofloxacin, norfloxacin, amoxicillin, tetracyclines (doxycycline), metronidazole, and trimethoprim-sulfamethoxazole are effective against small intestinal bacterial overgrowth (SIBO). |
| Colon and anorectal | stimulant laxatives and stool softeners for constipation management. |
Cutaneous manifestations management[edit | edit source]
Current medicines are restricted and inadequate in treating scleroderma's cutaneous symptoms. Autologous fat transfer (AFT) is a surgical procedure for face rejuvenation that has been used for many decades. Adipose stem cells (ASCs) found in fat grafts have also showed promise in terms of anti-inflammatory and regenerative characteristics. AFT has recently been repurposed to treat systemic sclerosis and localised scleroderma skin symptoms. AFT appears to enhance mouth and hand functions, Raynaud's symptoms, and digital ulcerations in scleroderma patients, according to research. AFT is a safe operation with little postoperative problems, making it a prospective option for scleroderma treatment. More research is needed to properly characterise the impact of fat grafts on the recipient site and to define fat transfer criteria in fibrotic skin diseases.[23]
Physical Therapy Management[edit | edit source]
Physiotherapists are core members of a multi-disciplinary rheumatology team and should be appropriately trained to work with patients and to manage the many different diseases presenting to the service.[24]
Clinical evaluation/ assessment[edit | edit source]
Before beginning rehabilitation, the physiotherapist must do a thorough assessment of the patient in order to develop a suitable therapeutic programme especially upper limb functional and ROM assessment as the hands are the most commonly involved part of the body in scleroderma, with symptoms such as edema, Raynaud's phenomenon, sclerodactyly producing pain, and joint ROM reduction. Symptoms that are commonly experienced by patients with SSc and have a major impact on carrying out everyday activities, like fatigue, pain, limitations in hand function, and decreased mobility.[25]
Assessments specific to scleroderma include:
- Health Assessment Questionnaire-Disability Index
- Scleroderma Health Assessment Questionnaire (SHAQ)[26]
- Hand mobility in scleroderma (HAMIS) test [27]
Exercise and splinting[edit | edit source]
Exercise is regarded as a non‐pharmaceutical intervention for people with SSc.Regular exercise training may provide anti-inflammatory effects in chronic conditions characterised by systemic low grade inflammation (for example, type 2 diabetes) by lowering inflammatory markers. Given the probable role of inflammation in the aetiology and clinical symptoms of SSc, if exercise training can reduce inflammation, it may be a useful intervention in controlling some problematic SSc symptoms, such as pain.[28]In general the exercises should emphasize flexion of the metacarpophalangeal joint,extension of the proximal interphalangeal joints, and flexion and abduction of the thumb. Stretching exercises for the hand were shown to increase motion and subsequent function in daily tasks.[29]Hand splints to increase joint motion must be used very carefully as dynamic splints were shown to exacerbate Raynaud’s. Static splints may be useful if patients have inflammation but should only be worn at night until the inflammation decreases.[29]
Exercise interventions can include: [28]
- Hand exercises.
- Mouth exercises.
- Aerobic exercise.
- Resistance exercise- should be started before there is any observed loss of motion.
- Range of motion exercise.
- Kinesiotherapy.
- Hydro Kinesiology .
- Recreational exercise (i.e. lawn bowls).
Modalities[edit | edit source]
Pulsed dye laser:
The concept of selective photothermolysis supports the treatment of telangiectasia with pulsed dye laser . Previous research using equivalent treatment parameters to those utilized herein discovered that telangiectasia responds effectively to pulsed dye laser, with the majority of cases clearing within one to two treatments.[30]
Heat therapy:
Heat modalities, such as paraffin, in conjunction with exercise programmes, have been shown to be effective in increasing or maintaining joint motion and hand function.[29]Using of paraffin in order to warm up the hands of patients with SSc has been introduced back in 1983 by Askew et al.[31] Few studies have examined the effect of paraffin on the hands of patients with SSc and they all found positive results such as an immediate improvement in patients’ range of motion of hands and fingers after warming up the hands in paraffin.[32]
Manual lymph drainage (MLD)[edit | edit source]
Manual lymph drainage (MLD) is the use of mild massage to the skin that promotes smooth muscle contraction around lymphatic vessels, increasing lymphatic flow and eliminating edema and extra interstitial fluid.Microvascular alterations, increased sympathetic activity, and inflammatory changes are all part of the complicated pathophysiology of edema in SSc. In addition to the recognized microvascular alterations in SSc, it has been observed that lymphatic circulation in the upper extremities is disrupted. The presence of finger and hand edema in SSc could support the MLD therapy approach.[33]Difficulties in daily living activities occur because of conditions such as hardness in the hand, the presence of edema pain, reduced grip strength and slower hand movements that develop in connection with systemic sclerosis. MLD was found to improve hand function and quality of life in SSc patients when added to the rehabilitation programme.There is also a reduction in edema, skin involvement, discomfort, and respiratory and sleep issues. [33]
Occupational Therapy (OT)[edit | edit source]
Occupational therapy may also be required to address independence in daily life activities. A home evaluation may be recommended to ensure that the environment is suitable for the individual's needs. To assist with common everyday tasks, assistive gadgets may be prescribed.[34]
An ergonomic study of the workstation may allow people with scleroderma to keep their jobs.[34]
References[edit | edit source]
- ↑ Meyer O. CREST syndrome. Ann Med Interne (Paris) [Internet]. 2002;153(3):183–8.
- ↑ Le C, Bedocs PM. Calcinosis Cutis. StatPearls Publishing; 2023.
- ↑ Nelson FRT, Blauvelt CT. The Hand and wrist. In: Nelson FRT, Blauvelt CT, editors. A Manual of Orthopaedic Terminology. Elsevier; 2015. p. 307–41.
- ↑ Telangiectasia (Spider Veins) [Internet]. Pennmedicine.org. [cited 2023 Sep 8]. Available from: https://www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/telangiectasia-spider-veins
- ↑ 5.0 5.1 Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol [Internet]. 2019;11:257–73.
- ↑ Wangkaew S, Euathrongchit J, Wattanawittawas P, Kasitanon N, Louthrenoo W. Incidence and predictors of interstitial lung disease (ILD) in Thai patients with early systemic sclerosis: Inception cohort study. Mod Rheumatol [Internet]. 2016;26(4):588–93.
- ↑ 7.0 7.1 Lescoat A, Varga J, Matucci-Cerinic M, Khanna D. New promising drugs for the treatment of systemic sclerosis: pathogenic considerations, enhanced classifications, and personalized medicine. Expert Opin Investig Drugs [Internet]. 2021;30(6):635–52.
- ↑ De Martinis M, Ciccarelli F, Sirufo MM, Ginaldi L. An overview of environmental risk factors in systemic sclerosis. Expert Rev Clin Immunol [Internet]. 2016;12(4):465–78.
- ↑ van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis and rheumatism [Internet]. 2013 [cited 2023 Sep 8];65(11).
- ↑ Hsu V, Varga J, Schlesinger N. Calcinosis in scleroderma made crystal clear. Curr Opin Rheumatol [Internet]. 2019;31(6):589–94.
- ↑ Chang SH, Jun JB, Lee YJ, Kang TY, Moon KW, Ju JH, et al. A clinical comparison of an endothelin receptor antagonist and phosphodiesterase type 5 inhibitors for treating digital ulcers of systemic sclerosis. Rheumatology (Oxford) [Internet]. 2021;60(12):5814–9.
- ↑ 12.0 12.1 1. Hughes M, Allanore Y, Chung L, Pauling JD, Denton CP, Matucci-Cerinic M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol [Internet]. 2020;16(4):208–21.
- ↑ 13.0 13.1 Li B, Yan J, Pu J, Tang J, Xu S, Wang X. Esophageal dysfunction in systemic sclerosis: An update. Rheumatol Ther [Internet]. 2021;8(4):1535–49.
- ↑ Adigun R, Goyal A, Hariz A. Systemic Sclerosis. StatPearls Publishing; 2022.
- ↑ 15.0 15.1 Foti R, De Pasquale R, Dal Bosco Y, Visalli E, Amato G, Gangemi P, et al. Clinical and histopathological features of Scleroderma-like disorders: An update. Medicina (Kaunas) [Internet]. 2021;57(11):1275.
- ↑ Halachmi S, Gabari O, Cohen S, Koren R, Amitai DB, Lapidoth M. Telangiectasis in CREST syndrome and systemic sclerosis: correlation of clinical and pathological features with response to pulsed dye laser treatment. Lasers Med Sci [Internet]. 2014;29(1):137–40.
- ↑ Joslin N. Early identification key to scleroderma treatment. Nurse Pract [Internet]. 2004;29(7):24–39; quiz 40–1.
- ↑ Gelber AC, Wigley FM. Disease severity as a predictor of outcome in scleroderma. Lancet [Internet]. 2002;359(9303):277–9.
- ↑ Hughes M, Allanore Y, Chung L, Pauling JD, Denton CP, Matucci-Cerinic M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol [Internet]. 2020;16(4):208–21.
- ↑ 20.0 20.1 Herrick AL, Philobos M. Pharmacological management of digital ulcers in systemic sclerosis - what is new? Expert Opin Pharmacother [Internet]. 2023;24(10):1159–70.
- ↑ Kempanna Y. P28 Recalcitrant exuberant digital calcinosis cutis in a patient of CREST syndrome - A case report. Rheumatol Adv Pract [Internet]. 2022;6(Supplement_1).
- ↑ Nassar M, Ghernautan V, Nso N, Nyabera A, Castillo FC, Tu W, et al. Gastrointestinal involvement in systemic sclerosis: An updated review. Medicine (Baltimore) [Internet]. 2022;101(45):e31780.
- ↑ Kawakibi AR, Khouri AN, Cederna PS, Strong AL. Novel indications for autologous fat grafting in reconstruction: scleroderma. Plast Aesthet Res [Internet]. 2023;10(0):48.
- ↑ Cohen A, Morrow H, Cleary G. Physiotherapy and rheumatological disorders. Paediatr Child Health (Oxford) [Internet]. 2014;24(2):83–8.
- ↑ Willems LM, Vriezekolk JE, Schouffoer AA, Poole JL, Stamm TA, Boström C, et al. Effectiveness of nonpharmacologic interventions in systemic sclerosis: A systematic review: Nonpharmacologic care in SSc. Arthritis Care Res (Hoboken) [Internet]. 2015;67(10):1426–39.
- ↑ Johnson SR, Hawker GA, Davis AM. The health assessment questionnaire disability index and scleroderma health assessment questionnaire in scleroderma trials: An evaluation of their measurement properties. Arthritis Rheum [Internet]. 2005;53(2):256–62.
- ↑ Sandqvist G, Eklund M. Hand mobility in scleroderma (HAMIS) test: The reliability of a novel hand function test. Arthritis Rheum [Internet]. 2000;13(6):369–74.
- ↑ 28.0 28.1 Frade S, Cameron M, Espinosa-Cuervo G, Suarez-Almazor ME, Lopez-Olivo MA. Exercise and physical therapy for systemic sclerosis. Cochrane Libr [Internet]. 2022;2022(3).
- ↑ 29.0 29.1 29.2 Krysia Dziedzic, Hammond A. Rheumatology : evidence-based practice for physiotherapists and occupational therapists. Edinburgh: Churchill Livingstone; 2010.
- ↑ Halachmi S, Gabari O, Cohen S, Koren R, Amitai DB, Lapidoth M. Telangiectasis in CREST syndrome and systemic sclerosis: correlation of clinical and pathological features with response to pulsed dye laser treatment. Lasers Med Sci [Internet]. 2014;29(1):137–40.
- ↑ Askew LJ, Backett VL, An K-N, Chao EYS. Objective evaluation of hand function in scleroderman patients to assess effectiveness of physical therapy. Rheumatology (Oxford) [Internet]. 1983;22(4):224–32.
- ↑ Kristensen LQ, Oestergaard LG, Bovbjerg K, Rolving N, Søndergaard K. Use of paraffin instead of lukewarm water prior to hand exercises had no additional effect on hand mobility in patients with systemic sclerosis: A randomized clinical trial. Hand Ther [Internet]. 2019;24(1):13–21.
- ↑ 33.0 33.1 Yılmaz A, Çalık BB, Kabul EG, Tasçı M, Çobankara V. Efficacy of manual lymph drainage in systemic sclerosis: A case report. Ann Clin Anal Med [Internet]. 2021;12(Suppl_03):3): S354.
- ↑ 34.0 34.1 Barange J. Case Study of Physiotherapy Treatment of a Patient with the Diagnosis Scleroderma.