Arterial Blood Gases: Difference between revisions
Kim Jackson (talk | contribs) m (Categorisation) |
(Added Technology Category) |
||
| (24 intermediate revisions by 5 users not shown) | |||
| Line 6: | Line 6: | ||
== Arterial Blood Gases == | == Arterial Blood Gases == | ||
[[Image:ABG.jpg|thumb|right|241x241px|Blood Gas Analyser]]Arterial blood gases (ABG's) is a blood test | [[Image:ABG.jpg|thumb|right|241x241px|Blood Gas Analyser]]Arterial blood gases (ABG's) is a blood test that is used to give an indication of ventilation, gas exchange, and acid-base status and is taken from an arterial blood supply<ref name="Hough">Hough A. Physiotherapy in Respiratory Care. An evidence-based approach to respiratory and cardiac management. 3rd ed. Cheltenham: Nelson Thomas Ltd. 2001</ref>. The arterial blood gas test is one of the most common tests performed on patients in intensive care units. At other levels of care, [[Pulse Oximeter|pulse oximetry]] plus transcutaneous carbon dioxide measurement is a less invasive alternative method of obtaining similar information.<ref name=":0">Scope health Arterial Gasometry: What is it? Why is it Necessary? Procedure, Compensation, Metabolic Disorders and Results Available: https://scopeheal.com/arterial-blood-gas/ (accessed 9.5.2022)</ref> | ||
To perform this test, blood is collected from a specific artery, usually the wrist's radial artery. This blood sample allows an accurate determination of the amount of oxygen that passes from the lungs to the blood. This test is the one most commonly performed to diagnose cases of [[Respiratory Failure|respiratory failure]]<ref name=":4">Well being pole Gasometry Available:https://wellbeingpole.com/gasometry/ (accessed 9.5.2022)</ref>.<br>Arterial blood gas test results can show if: | |||
* Lungs are getting enough oxygen. | |||
* Lungs are removing enough carbon dioxide. | |||
* Kidneys are working properly.<ref name=":0" /> | |||
== | == Uses == | ||
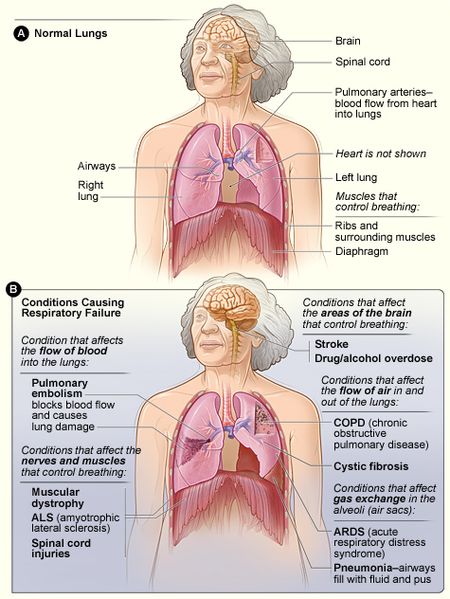
ABGs are very useful for detecting conditions that cause respiratory failure. Including: [[Respiratory Failure|Lung Failure]]; [[Acute Respiratory Distress Syndrome (ARDS)|Acute respiratory distress syndrome (ARDS)]]; [[Sepsis]]; Diabetic [[ketoacidosis]] (DKA); [[Cystic Fibrosis|Cystic fibrosis]]; [[Pneumonia]]; [[Emphysema]]; [[Shock|Hypovolemic shock]]; [[Heart Failure|Acute heart failure]]; [[Cardiac Arrest|Cardiac arrest]]; [[Chronic Kidney Disease|Kidney Failure]]; [[Sepsis|Septic Shock]]; [[Trauma-Informed Care|Trauma]]; Chronic vomiting; [[Diabetes|Uncontrolled diabetes]]; [[Asthma]] ; [[Chronic Obstructive Pulmonary Disease Rehabilitation Class|Chronic Obstructive Pulmonary Disease (COPD)]]; Hemorrhage; Drug Overdose; Metabolic Disease; Chemical Poisoning; To check if lung condition treatments are working.<ref name=":1" /> | |||
[[File:Respiratory failure.jpg|center|frameless|599x599px]] | |||
<ref name=":4" /> | |||
== | == Measurements == | ||
The key components of an ABG are: | |||
# pH - This measures the balance of acids and bases in the blood. | |||
# Partial pressure of oxygen (PaO2) - This measures the pressure of oxygen dissolved in the blood. | |||
# Partial pressure of carbon dioxide (PaCO2) - This measures the amount of carbon dioxide in the blood and how well carbon dioxide can move out of the lungs. | |||
# Bicarbonate (HCO3) - This is calculated using the measured values of pH and PaCO2 to determine the amount of the primary compound made from carbon dioxide (CO2.) | |||
# Oxygen saturation (O2 Sat) - This measures how much hemoglobin in the blood is carrying oxygen. | |||
# Oxygen content (O2CT) - This measures the amount of oxygen in the blood. | |||
# Hemoglobin - This measures the amount of hemoglobin in the blood. | |||
== Normative Values == | |||
According to the National Institute of Health, typical normal values are: | |||
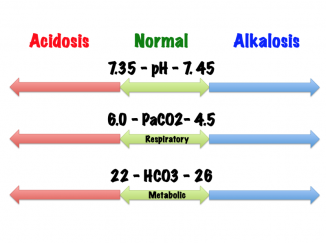
* pH: 7.35-7.45 | |||
* Partial pressure of oxygen (PaO2): 75 to 100 mmHg | |||
* Partial pressure of carbon dioxide (PaCO2): 35-45 mmHg | |||
* Bicarbonate (HCO3): 22-26 mEq/L | |||
* Oxygen saturation (O2 Sat): 94-100%<ref name=":1">Nurse org. ABG test Available:https://nurse.org/articles/arterial-blood-gas-test/ (accessed 9.5.2022)</ref> | |||
* | |||
* | |||
== Interpretation of ABGs == | == Interpretation of ABGs == | ||
| Line 181: | Line 45: | ||
#* Decreased = Acidosis | #* Decreased = Acidosis | ||
# <u>Look at the PaCO2</u> | # <u>Look at the PaCO2</u> | ||
# | #* Increased = Respiratory Acidosis | ||
# | #* Decreased = Respiratory Alkalosis | ||
# <u>Look at the HCO3</u> | # <u>Look at the HCO3</u> | ||
#* Increased = Metabolic Alkalosis | #* Increased = Metabolic Alkalosis | ||
#* Decreased = Metabolic Acidosis | #* Decreased = Metabolic Acidosis | ||
# <u>Look at the O2</u> | # <u>Identify if there is a compensation</u> | ||
#* Full compensation if the pH is within the normal range | |||
#* Partial compensation if either the PaCO2 or HCO3 value is wavering to compensate for the primary acid-base disturbance; but, the pH is still not within the normal physiologic range. | |||
# <u>Look at the O2</u><br> | |||
'''The results should always be read and compared in reference to the patient's previous ABG (if available) as you will then be able to assess a trend and make a more accurate assessment on whether you should treat or if your treatment has been successful or not.''' | |||
== | == Primary Acid-base disturbances == | ||
They are: | |||
# '''Uncompensated Respiratory Acidosis:''' This occurs when there is an increase in the PaCO2 level without a resultant alteration (increase) of the HCO3 value. Thus, there will an acidosis due to respiratory failure (inability to remove excess carbondioxide from the blood and the lungs). | |||
# '''Partially compensated Respiratory Acidosis:''' This occurs when there is an increase in the PaCO2 level with a resultant alteration (increase) of the HCO3 value; but, the pH is still not within the normal range. Thus, there will still be acidosis due to respiratory failure (inability to remove excess carbondioxide from the blood and the lungs). | |||
# | # '''Fully compensated Respiratory Acidosis:''' This occurs when there is an increase in the PaCO2 level with a resultant alteration (increase) of the HCO3 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the acidosis due to respiratory failure (inability to remove excess carbondioxide from the blood and the lungs) with metabolic alkalosis. | ||
# '''Uncompensated Respiratory Alkalosis:''' This occurs when there is a decrease in the PaCO2 level without a resultant alteration (decrease) of the HCO3 value. Thus, there will be an alkalosis due to respiratory failure (excess carbondioxide exhalation from the lungs and reduced carbondioxide tension in the blood). | |||
# '''Partially compensated Respiratory Alkalosis:''' This occurs when there is a decrease in the PaCO2 level with a resultant alteration (decrease) of the HCO3 value; but, the pH is still not within the normal range. Thus, there will be an alkalosis due to respiratory failure (excess carbondioxide exhalation from the lungs and reduced carbondioxide tension in the blood). | |||
# '''Fully compensated Respiratory Alkalosis:''' This occurs when there is a decrease in the PaCO2 level with a resultant alteration (decrease) of the HCO3 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the alkalosis due to respiratory failure (excess carbondioxide exhalation from the lungs and reduced carbondioxide tension in the blood) with metabolic acidosis. | |||
# '''Uncompensated Metabolic Acidosis:''' This occurs when there is an decrease in the HCO3 level without a resultant alteration (decrease) of the PaCO2 value. Thus, there will an acidosis due to metabolic failure (inability of the kidney to retain adequate bicarbonate). | |||
# '''Partially compensated Metabolic Acidosis:''' This occurs when there is a decrease in the HCO3 level with a resultant alteration (decrease) of the PaCO2 value; but, the pH is still not within the normal range. Thus, there will still be acidosis due to metabolic failure (inability of the kidney to retain adequate bicarbonate). | |||
# '''Fully compensated Metabolic Acidosis:''' This occurs when there is a decrease in the HCO3 level with a resultant alteration (decrease) of the PaCO2 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the acidosis due to metabolic failure (inability of the kidney to retain adequate bicarbonate) with respiratory alkalosis. | |||
# '''Uncompensated Metabolic Alkalosis:''' This occurs when there is a increase in the HCO3 level without a resultant alteration (increase) of the PaCO2 value. Thus, there will be an alkalosis due to metabolic failure (inability of the kidney to excrete excess bicarbonate). | |||
# '''Partially compensated Metabolic Alkalosis:''' This occurs when there is an increase in the HCO3 level with a resultant alteration (increase) of the PaCO2 value; but, the pH is still not within the normal range. Thus, there will be an alkalosis due to metabolic failure (inability of the kidney to excrete excess bicarbonate). | |||
# '''Fully compensated Metabolic Alkalosis:''' This occurs when there is an increase in the HCO3 level with a resultant alteration (increase) of the PaCO2 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the alkalosis due to metabolic failure (inability of the kidney to excrete excess bicarbonate) with respiratory acidosis. | |||
# '''Mixed Acid-Base disturbances:''' This occurs when there is either both metabolic and respiratory acidosis present or metabolic and respiratory alkalosis present at the same time of analysing arterial blood gases | |||
''' | |||
== Tutorials == | == Tutorials == | ||
| Line 215: | Line 80: | ||
<div class="col-md-4">{{#ev:youtube|kfJws8NQW1k|250}} <div class="text-right"><ref>ABGs (Arterial Blood Gas). Available from: https://www.youtube.com/watch?v=kfJws8NQW1k[last accessed 27/03/18]</ref></div></div> | <div class="col-md-4">{{#ev:youtube|kfJws8NQW1k|250}} <div class="text-right"><ref>ABGs (Arterial Blood Gas). Available from: https://www.youtube.com/watch?v=kfJws8NQW1k[last accessed 27/03/18]</ref></div></div> | ||
</div> | </div> | ||
== Useful Resources == | == Useful Resources == | ||
[http://www.prognosis.org/abg/arterial_blood_gas_calculator.php ABG Calculator] | [http://www.prognosis.org/abg/arterial_blood_gas_calculator.php ABG Calculator] | ||
[https://sites.google.com/site/doctorkipp/ Acid-Base Questions] | [https://sites.google.com/site/doctorkipp/ Acid-Base Questions] | ||
[http://www.adamw.org/med/apps/abg.cgi ABG Interpretation Quiz] | [http://www.adamw.org/med/apps/abg.cgi ABG Interpretation Quiz] | ||
[https://www.medistudents.com/osce-skills/arterial-blood-gases Steps to Perform an ABG Test] | |||
== References == | == References == | ||
| Line 242: | Line 96: | ||
[[Category:Respiratory]] | [[Category:Respiratory]] | ||
[[Category:Cardiopulmonary]] | [[Category:Cardiopulmonary]] | ||
[[Category: | [[Category:Cardiovascular System - Assessment and Examination]] | ||
[[Category:Assessment and Examination | [[Category:Respiratory System - Assessment and Examination]] | ||
[[Category:Technology]] | |||
Latest revision as of 15:47, 11 March 2024
Original Editor - Scott Buxton
Top Contributors - Adam Vallely Farrell, Kim Jackson, Uchechukwu Chukwuemeka, Scott Buxton, Lucinda hampton, Rachael Lowe, Admin, Joao Costa, 127.0.0.1, Laura Ritchie, Naomi O'Reilly, Abbey Wright and Angeliki Chorti
Arterial Blood Gases[edit | edit source]
Arterial blood gases (ABG's) is a blood test that is used to give an indication of ventilation, gas exchange, and acid-base status and is taken from an arterial blood supply[1]. The arterial blood gas test is one of the most common tests performed on patients in intensive care units. At other levels of care, pulse oximetry plus transcutaneous carbon dioxide measurement is a less invasive alternative method of obtaining similar information.[2]
To perform this test, blood is collected from a specific artery, usually the wrist's radial artery. This blood sample allows an accurate determination of the amount of oxygen that passes from the lungs to the blood. This test is the one most commonly performed to diagnose cases of respiratory failure[3].
Arterial blood gas test results can show if:
- Lungs are getting enough oxygen.
- Lungs are removing enough carbon dioxide.
- Kidneys are working properly.[2]
Uses[edit | edit source]
ABGs are very useful for detecting conditions that cause respiratory failure. Including: Lung Failure; Acute respiratory distress syndrome (ARDS); Sepsis; Diabetic ketoacidosis (DKA); Cystic fibrosis; Pneumonia; Emphysema; Hypovolemic shock; Acute heart failure; Cardiac arrest; Kidney Failure; Septic Shock; Trauma; Chronic vomiting; Uncontrolled diabetes; Asthma ; Chronic Obstructive Pulmonary Disease (COPD); Hemorrhage; Drug Overdose; Metabolic Disease; Chemical Poisoning; To check if lung condition treatments are working.[4]
Measurements[edit | edit source]
The key components of an ABG are:
- pH - This measures the balance of acids and bases in the blood.
- Partial pressure of oxygen (PaO2) - This measures the pressure of oxygen dissolved in the blood.
- Partial pressure of carbon dioxide (PaCO2) - This measures the amount of carbon dioxide in the blood and how well carbon dioxide can move out of the lungs.
- Bicarbonate (HCO3) - This is calculated using the measured values of pH and PaCO2 to determine the amount of the primary compound made from carbon dioxide (CO2.)
- Oxygen saturation (O2 Sat) - This measures how much hemoglobin in the blood is carrying oxygen.
- Oxygen content (O2CT) - This measures the amount of oxygen in the blood.
- Hemoglobin - This measures the amount of hemoglobin in the blood.
Normative Values[edit | edit source]
According to the National Institute of Health, typical normal values are:
- pH: 7.35-7.45
- Partial pressure of oxygen (PaO2): 75 to 100 mmHg
- Partial pressure of carbon dioxide (PaCO2): 35-45 mmHg
- Bicarbonate (HCO3): 22-26 mEq/L
- Oxygen saturation (O2 Sat): 94-100%[4]
Interpretation of ABGs[edit | edit source]
- Look at the pH
- Increased = Alkalosis
- Decreased = Acidosis
- Look at the PaCO2
- Increased = Respiratory Acidosis
- Decreased = Respiratory Alkalosis
- Look at the HCO3
- Increased = Metabolic Alkalosis
- Decreased = Metabolic Acidosis
- Identify if there is a compensation
- Full compensation if the pH is within the normal range
- Partial compensation if either the PaCO2 or HCO3 value is wavering to compensate for the primary acid-base disturbance; but, the pH is still not within the normal physiologic range.
- Look at the O2
The results should always be read and compared in reference to the patient's previous ABG (if available) as you will then be able to assess a trend and make a more accurate assessment on whether you should treat or if your treatment has been successful or not.
Primary Acid-base disturbances[edit | edit source]
They are:
- Uncompensated Respiratory Acidosis: This occurs when there is an increase in the PaCO2 level without a resultant alteration (increase) of the HCO3 value. Thus, there will an acidosis due to respiratory failure (inability to remove excess carbondioxide from the blood and the lungs).
- Partially compensated Respiratory Acidosis: This occurs when there is an increase in the PaCO2 level with a resultant alteration (increase) of the HCO3 value; but, the pH is still not within the normal range. Thus, there will still be acidosis due to respiratory failure (inability to remove excess carbondioxide from the blood and the lungs).
- Fully compensated Respiratory Acidosis: This occurs when there is an increase in the PaCO2 level with a resultant alteration (increase) of the HCO3 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the acidosis due to respiratory failure (inability to remove excess carbondioxide from the blood and the lungs) with metabolic alkalosis.
- Uncompensated Respiratory Alkalosis: This occurs when there is a decrease in the PaCO2 level without a resultant alteration (decrease) of the HCO3 value. Thus, there will be an alkalosis due to respiratory failure (excess carbondioxide exhalation from the lungs and reduced carbondioxide tension in the blood).
- Partially compensated Respiratory Alkalosis: This occurs when there is a decrease in the PaCO2 level with a resultant alteration (decrease) of the HCO3 value; but, the pH is still not within the normal range. Thus, there will be an alkalosis due to respiratory failure (excess carbondioxide exhalation from the lungs and reduced carbondioxide tension in the blood).
- Fully compensated Respiratory Alkalosis: This occurs when there is a decrease in the PaCO2 level with a resultant alteration (decrease) of the HCO3 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the alkalosis due to respiratory failure (excess carbondioxide exhalation from the lungs and reduced carbondioxide tension in the blood) with metabolic acidosis.
- Uncompensated Metabolic Acidosis: This occurs when there is an decrease in the HCO3 level without a resultant alteration (decrease) of the PaCO2 value. Thus, there will an acidosis due to metabolic failure (inability of the kidney to retain adequate bicarbonate).
- Partially compensated Metabolic Acidosis: This occurs when there is a decrease in the HCO3 level with a resultant alteration (decrease) of the PaCO2 value; but, the pH is still not within the normal range. Thus, there will still be acidosis due to metabolic failure (inability of the kidney to retain adequate bicarbonate).
- Fully compensated Metabolic Acidosis: This occurs when there is a decrease in the HCO3 level with a resultant alteration (decrease) of the PaCO2 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the acidosis due to metabolic failure (inability of the kidney to retain adequate bicarbonate) with respiratory alkalosis.
- Uncompensated Metabolic Alkalosis: This occurs when there is a increase in the HCO3 level without a resultant alteration (increase) of the PaCO2 value. Thus, there will be an alkalosis due to metabolic failure (inability of the kidney to excrete excess bicarbonate).
- Partially compensated Metabolic Alkalosis: This occurs when there is an increase in the HCO3 level with a resultant alteration (increase) of the PaCO2 value; but, the pH is still not within the normal range. Thus, there will be an alkalosis due to metabolic failure (inability of the kidney to excrete excess bicarbonate).
- Fully compensated Metabolic Alkalosis: This occurs when there is an increase in the HCO3 level with a resultant alteration (increase) of the PaCO2 value; thereby, balancing the pH within the normal range. Thus, there will be compensation for the alkalosis due to metabolic failure (inability of the kidney to excrete excess bicarbonate) with respiratory acidosis.
- Mixed Acid-Base disturbances: This occurs when there is either both metabolic and respiratory acidosis present or metabolic and respiratory alkalosis present at the same time of analysing arterial blood gases
Tutorials[edit | edit source]
Useful Resources[edit | edit source]
References[edit | edit source]
- ↑ Hough A. Physiotherapy in Respiratory Care. An evidence-based approach to respiratory and cardiac management. 3rd ed. Cheltenham: Nelson Thomas Ltd. 2001
- ↑ 2.0 2.1 Scope health Arterial Gasometry: What is it? Why is it Necessary? Procedure, Compensation, Metabolic Disorders and Results Available: https://scopeheal.com/arterial-blood-gas/ (accessed 9.5.2022)
- ↑ 3.0 3.1 Well being pole Gasometry Available:https://wellbeingpole.com/gasometry/ (accessed 9.5.2022)
- ↑ 4.0 4.1 Nurse org. ABG test Available:https://nurse.org/articles/arterial-blood-gas-test/ (accessed 9.5.2022)
- ↑ https://ed.ted.com/on/9q9pS35Z
- ↑ Arterial Blood Gas (ABG) Tic-Tac-Toe Examples. Available from: https://www.youtube.com/watch?v=_OpvyEIlFj8[last accessed 27/03/18]
- ↑ 6 Easy Steps to ABG Analysis. Available from: https://www.youtube.com/watch?v=WUf-cPpnrXw[last accessed 27/03/18]
- ↑ ABGs (Arterial Blood Gas). Available from: https://www.youtube.com/watch?v=kfJws8NQW1k[last accessed 27/03/18]