Myasthenia Gravis
Original Editor - Wendy Walker
Top Contributors - Garima Gedamkar, Laura Ritchie, Khloud Shreif, Wendy Walker, Nikhil Benhur Abburi, Sheik Abdul Khadir, Rachael Lowe, Evan Thomas, Kim Jackson, Lucinda hampton, Admin, Saeed Dokhnan, WikiSysop, Candace Goh and Sai Kripa
This article or area is currently under construction and may only be partially complete. Please come back soon to see the finished work! (11/10/2021)
Historical Aspect[edit | edit source]
Myasthenia Gravis (MG) was first reported of an American Chief Opechancanough, died in 1664 and historical chroniclers from Virginia described it as “the excessive fatigue he encountered wrecked his constitution; his flesh became macerated; the sinews lost their tone and elasticity, and his eyelids were so heavy that he could not see unless they were lifted up by his attendants, he was unable to walk, but his spirit rising above the ruins of his body directed from the litter on which he was carried by his Indians”.
A patient with “fatigable weakness” involving ocular and bulbar muscles was described by peers of Willis in 1672 as “spurious palsy”. In 1879, the first paper about this disease described three cases of myasthenia gravis with features of bilateral ptosis, diplopia, dysphagia, facial paresis, and neck muscles weakness. Later on, a complete description of MG including the severity, prognosis, and different presentations of the cases was presented by Samuel Goldflam in 1893.
In 1895, The phenomenon that known later under the name of “Mary Walker effect” was first reported by Jolly, at the Berlin Society meeting, This phenomenon was reported in 1938 as “if you stimulate one group of muscles to exhaustion, weakness is apparent in muscles that are not stimulated; an evidence of a circulating factor causing neuromuscular weakness”. In 1959-1960, Myasthenia Gravis was supposed to have autoimmune etiology.[1]
Definition[edit | edit source]
Myasthenia Gravis is a relatively rare, long term condition and an autoimmune neuromuscular disease caused by circulating antibodies that block acetylcholine receptors at the postsynaptic neuromuscular junction, inhibiting the excitatory effects of the neurotransmitter acetylcholine on nicotinic receptors at neuromuscular junctions leading to fluctuating muscle weakness and fatigue which is more prominent in the afternoon. It is generalized muscle weakness and the most common muscles affected are muscles that control the eyes and eyelids, facial expressions, chewing, swallowing, and speaking, respiratory muscles can be affected and may require urgent intervention. MG affects people at any age but it is common to affect women under 40[3].
Clinically Relevant Anatomy[edit | edit source]
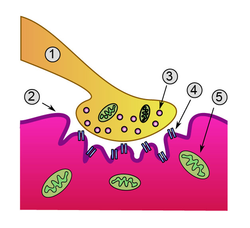
Detailed view of a neuromuscular junction:
- Presynaptic terminal
- Sarcolemma
- Synaptic vesicle
- Nicotinic acetylcholine receptor
- Mitochondrion
Epidemiology[edit | edit source]
Incidence rate of MG estimates to be about 5 to 30 cases per million person years, and prevalence rate to be between 10 to 20 cases per 100,000 population. There is equal geographic distribution in the incidence and prevalence of MG in both adults and children and juvenile MG that start before the age of 18 years is almost 10% of all cases of MG[4].
Its prevalence has been increasing over the past several decades secondary to better awareness, recognition and increased survival, it s not a linear increase, the age of onset is characterized by a bimodal distribution with an early incidence peak in the 2nd to 3rd decades affecting young women and a late peak in the 6th to 8th decades that is primarily seen in men[5].[6]
Mechanism of Injury / Pathological Process[edit | edit source]
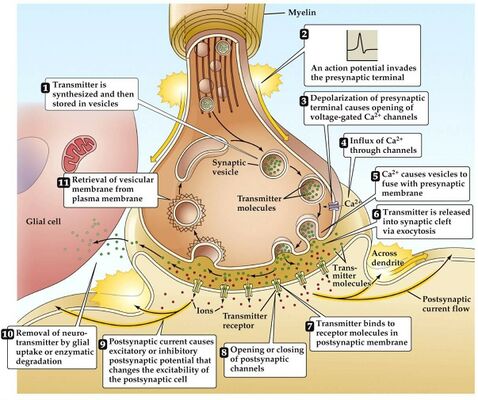
In Myasthenia gravis (MG) antibodies form against nicotinic acetylcholine (ACh) postsynaptic receptors at the neuromuscular junction (NMJ) of the skeletal muscles[7].The basic pathology is a reduction in the number of ACh receptors (AChRs) at the postsynaptic muscle membrane brought about by an acquired autoimmune reaction producing anti-AChR antibodies[8].
During normal conditions of depolarization Calcium channels on the presynaptic membrane open and triggering release of ACh-R into the synaptic cleft when it reaches binds to postsynaptic membrane receptors where the end-plate potential (EPP) generated, this potential is larger than the threshold needed to generate postsynaptic action potential. In MG patients there is decrease in number or activity of ACh-R so decrease in the EPP, hence this potential may be sufficient at rest but with activities there is not sufficient EPP to trigger action potential.
Clinical Presentation[edit | edit source]
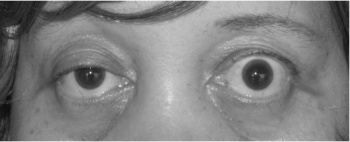
- The usual initial complaint is a specific muscle weakness rather than generalised weakness - frequently ocular (eye) symptoms.
- Extraocular muscle weakness or ptosis is present initially in 50% of patients and occurs during the course of illness in 90% of patients. Patients also frequently report diploplia (double vision).
- The disease remains exclusively ocular in 10 - 40% of patients.
- Rarely, patients have generalised weakness without ocular muscle weakness.
- Eye muscle weakness are often asymmetrical and variable.
- Bulbar muscle weakness is also common, fascial muscle weakness can result in, trouble smiling or trouble whistling.
- Weakness tends to spread from the ocular to facial to bulbar muscles and then to truncal and limb muscles.
- With weakness of neck flexors more common and neck extensor weakness are rare to present.
- Limb weakness may be more severe proximally than distally.
- Isolated limb muscle weakness is the presenting symptom in fewer than 10% of patients.
- Weakness is typically least severe in the morning and worsens as the day progresses.
- Weakness is increased by exertion and alleviated by rest.
- Weakness progresses from mild to more severe over weeks or months, with exacerbations and remissions.
- About 87% of patients have generalised disease within 13 months after onset, and about 75% will have generalized weakness within the first 2 to 3 years.
- Less often, symptoms may remain limited to the extra-ocular and eyelid muscles for many years.
- Respiratory muscle weakness develop in up to 40% of patients and myasthenic crisis( respiratory failure) present in about 15%- 20% of patients with MG[4].
Classification[edit | edit source]
Subtypes of MG are broadly classified as follows:[1]
- Early-onset MG: age at onset <50 years. Thymic hyperplasia, usually females,
- Late-onset MG: age at onset >50 years. Thymic atrophy, mainly males,
- Thymoma-associated MG (10%–15%)
- MG with anti-MUSK antibodies,
- Ocular MG (oMG): symptoms only affecting extraocular muscles,
- MG with no detectable AChR and muscle-specific tyrosine kinase (MuSK) antibodies.
The most widely utilised classification of MG is the Myasthenia Gravis Foundation of America Clinical Classification[9]
- Class I: Any ocular muscle weakness, possible ptosis, no other evidence of muscle weakness elsewhere
- Class II: Mild weakness affecting other than ocular muscles; may also have ocular muscle weakness of any severity
- Class IIa: Predominantly affecting limb, axial muscles, or both; may also have lesser involvement of oropharyngeal muscles
- Class IIb: Predominantly bulbar and/or respiratory muscles; may also have lesser or equal involvement of limb, axial muscles, or both
- Class III: Moderate weakness affecting other than ocular muscles; may also have ocular muscle weakness of any severity
- Class IIIa: Predominantly affecting limb, axial muscles, or both; may also have lesser involvement of oropharyngeal muscles
- Class IIIb: Predominantly bulbar and/or respiratory muscles; may also have lesser or equal involvement of limb, axial muscles, or both
- Class IV: Severe weakness affecting other than ocular muscles; may also have ocular muscle weakness of any severity
- Class IVa: Predominantly affecting limb, axial muscles, or both; may also have lesser involvement of oropharyngeal muscles
- Class IVb: Predominantly bulbar and/or respiratory muscles; may also have lesser or equal involvement of limb, axial muscles, or both (Can also include feeding tube without intubation)
- Class V: Intubation needed to maintain airway, with or without mechanical ventilation
Diagnostic Procedures[edit | edit source]
The diagnosis of myasthenia gravis based on the associated and confirmed symptoms and signs the patient presented with plus a positive test for specific autoantibodies, that are important to detect MG and define MG subgroups.
1- The most commonly immunological tets:
Antibodies against acetylcholine receptors (Anti- AchR) is heighly specific for MG, Anti- AchR not used to predict the severity of the disease, negative test results (Seronegativity) may occur if the test is done early or if it is immunosuppression condition. It has sesitivity approximately 85% for gMG and 50% for oMG.
Muscle-specific kinase antibodies antibodies.
Antibodies against striated muscle cytoplasmic proteins: found in thymoma patients with and without myasthenia gravis.
2- Tensilon (Edrophonium Chloride) Test: pharmacological test used for the diagnosis of certain neural diseases, mainly MG and distinguish Myasthenia crisiss from cholinergic crisis. It is An Ach-R inhibitor that prolong the duration of Ach in NMJ.
Procedure: it is administered intravenously IV while the patient is observed for any objective improvement in muscle strength specially movement f extraocular muscles or improve in ptosis, while asymmetrical improvement in eyelid muscles considered appositive result for the test. Durning this test the patients must be monitored for cardiac and blood pressure prior to injection because of possible risk of arrhythmia and hypotension.
Side effects: increase in salivation, sweating, nausea, stomach cramping, and muscle fasciculation. While hypotension and and bradycardia are un common side effect and relief with rest.
Sensitivity: Tensilon test has a sensitivity of 71.5%–95% for the diagnosis of MG.
3- Ice Pack Test: non pharmacological test used when Edrophonium Chloride test is contarindicated, used for patients with ptosis. Apply an ice pack over the eye for 2–5 minutes and assessing for improvement in ptosis.
4- Electrophysiological Tests: using repetitive nerve stimulation study and single fiber electromyography are the main electrophysiologic tests for diagnosis of MG. The nerve stimulated supramaximally at 2-3 Hz, and the decrement between the first and the fifth evoked muscle action potential is diagnostic for MG. It is abnormal in approximately 75% of patients with generalized myasthenia gravis and 50% of patients with Ocular myasthenia gravis (oMG).
Single-fiber electromyography (SFEMG) : it uses a needle electrode to identify AP form individual ms fibers and can record action potential of muscle fibers innervated by the same motor axon. Although it is heighly sensitive test but abnormality didn’t mean it is necessarly to be MG that may be another motor neuron disease, polymyositis, or peripheral neuropathy
Sensitivity: the sensitivity of this test is approximately 85% for gMG and 50% for oMG[1][10] .
Differential Diagnosis[edit | edit source]
- Amyotrophic Lateral Sclerosis
- Basilar Artery Thrombosis
- Brainstem Gliomas
- Cavernous Sinus Syndromes
- Dermatomyositis/Polymyositis
- Lambert-Eaton Myasthenic Syndrome
- Multiple Sclerosis
- Myocardial Infarction
- Pulmonary Embolism
- Sarcoidosis and Neuropathy
- Thyroid Disease
- Tolosa-Hunt Syndrome
Outcome Measures[edit | edit source]
Management / Interventions[edit | edit source]
Medical Management[edit | edit source]
There are four basic therapies used to treat MG:
- symptomatic treatment with acetylcholinesterase inhibitors,
- rapid short-term immunomodulating treatment with plasmapheresis and intravenous immunoglobulin,
- chronic long-term immunomodulating treatment with glucocorticoids and other immunosuppressive drugs,
- surgical treatment
Surgical Management (Thymectomy)[edit | edit source]
Surgical treatment is strongly recommended for patients with thymoma. The clinical efficacy of thymectomy in other situations has been questioned because the evidence supporting its use is not solid. Surgical treatment is strongly recommended for patients with thymoma. The benefit of thymectomy evolves over several years. Thymectomy is advised as soon as the patient’s degree of weakness is sufficiently controlled to permit surgery. Patients undergoing surgery are usually pretreated with low-dose glucocorticoids and I V Ig. Thymectomy may not be a viable therapeutic approach for anti-MuSK antibody-positive patients because their thymi lack the germinal centres and infiltrate of lymphocytes that characterize thymi in patients who have anti-AChR antibodies. This supports a different pathologic mechanism in anti-MuSK Ab-positive and anti-AChR Ab-positive MG. Most experts consider thymectomy to be a therapeutic option in anti-AChR Ab-positive gMG with disease onset before the age of 50 years.
Physiotherapy Management[edit | edit source]
Rehabilitation alone or in combination with other forms of treatment can relieve or reduce symptoms for some people with MG.
MG patients should find the optimal balance between physical activity and rest. It is not possible to cure the weakness by active physical training. However, most MG patients are more passive than they need to be. Physical activity and physical training of low to medium intensity is recommended[11].
One study showed a clear benefit from a strength training exercise program for a group of patients with mild to moderate MG[12], concluding "physical training can be carried out safely in mild MG and provides some improvement of muscle force".
Physical exercise is well tolerated in patients with well-regulated Myasthenia Gravis. Aerobic exercise and mild strength training can be advised and should be supervised.[13]
Balance strategy training maybe effective in improving balance and more research into this domain has to be done[14]
General advice for exercise programs for people with MG:
- Aim to strengthen large muscle groups, particularly proximal muscles of shoulders and hips
- Advise patient to do the exercises at their "best time of day" ie. when not feeling tired - for the majority of MG patients this will be morning
- If a patient is taking pyridostigmine, exercise at peak dose ie. 1.5 to 2 hours after taking a dose
- Moderate intensity of exercise only: the patient should not experience worsening of MG symptoms (eg. ptosis or diplopia) during exercise
- General aerobic exercise is also valuable, helping with respiratory function as well stamina
Myasthenic Crisis[edit | edit source]
Myasthenic crisis is defined as respiratory muscle weakness that is severe enough to necessitate intubation or delay extubation. Prognosis in myasthenic crisis has dramatically improved over the last 4 decades from a mortality rate of 75% to the current 4.5%. It is prudent to closely observe MG patients with respiratory difficulty in a supervised setting. The forced vital capacity (FVC) and the negative inspiratory force (NIF) are the main respiratory parameters for monitoring, and both should be measured frequently during the hospital admission. Abnormalities of arterial blood gases are insensitive measures of respiratory muscle weakness as they often are late-occurring abnormalities only after the onset of life-threatening respiratory failure. Elective intubation should be considered if serial FVC measurements show values less than 20 mL/kg or if the NIF is less than 30 cm H2O.[15] Rapid induction therapies such as plasmapheresis or IVIg should be considered. In most cases, the initiation or maintenance of high-dose corticosteroids is also necessary.
Prognosis[edit | edit source]
With treatment, people with MG have a normal life expectancy. Some combination of medication, thymectomy, and other therapies enable most myasthenics to lead normal or near-normal lives. Sometimes people experience remission. However, for some people quality of life is affected significantly - either by the severity of the disease or severity of side effects from the medication. Generally, those who are quickly diagnosed and receive effective treatment have the best outcomes.
Medical treatment involves the use of anticholinesterase agents, immunosuppressive drugs, plasmapheresis, and gammaglobulin, with reported complete clinical remission rates (CCRRs) as low as 15%. Accordingly, thymectomy has become an increasingly accepted procedure for the treatment of MG, as it can achieve CCRRs as high as 80% in accordance with most of the reports published in the literature.
Resources[edit | edit source]
The Myasthenia Gravis Foundation of America (MGFA) has a comprehensive website
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Annapurni Jayam Trouth et. al. Autoimmune DiseasesfckLRVolume 2012 (2012), Article ID 874680, 10 pages
- ↑ Osmosis. Myasthenia gravis - causes, symptoms, treatment, pathology. Available from: http://www.youtube.com/watch?v=bYGxGdu9MsQ[last accessed 10/9/2021]
- ↑ Gilhus NE, Socrates T, Amelia E, Jacqueline P, Burns TM. Myasthenia gravis (Primer). Nature Reviews: Disease Primers. 2019;5(1).
- ↑ 4.0 4.1 Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurologic clinics. 2018 May 1;36(2):253-60.
- ↑ McGrogan A, Sneddon S, De Vries CS. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology. 2010;34(3):171-83.
- ↑ Keesey JC. Clinical evaluation and management of myasthenia gravis. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2004 Apr;29(4):484-505.
- ↑ Strauss AJ, Seegal BC, Hsu KC, Burkholder PM, Nastuk WL, Osserman KE. Immunofluorescence demonstration of a muscle binding, complement-fixing serum globulin fraction in myasthenia gravis. Proceedings of the Society for Experimental Biology and Medicine. 1960 Oct;105(1):184-91.
- ↑ Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871-2.
- ↑ Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. Jul 12 2000;55(1):16-23
- ↑ Sieb JP. Myasthenia gravis: an update for the clinician. Clinical & Experimental Immunology. 2014 Mar;175(3):408-18.
- ↑ Skeie GO, Apostolski S, Evoli A et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur. J. Neurol.17,893–902 (2010)
- ↑ Lohi EL1, Lindberg C, Andersen O. Physical training effects in myasthenia gravis. Arch Phys Med Rehabil. 1993 Nov;74(11):1178-80
- ↑ Westerberg, Molin CJ, Lindblad I, Emtner M, Punga AR. Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: A pilot study. Muscle Nerve 56: 207-214, 2017
- ↑ S H Wong, J Nitz, K Williams, S Brauer Effects of balance strategy training in myasthenia gravis: a case study series May 2015 Volume 101, Supplement 1, Pages e1657–e1658
- ↑ Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment [published online ahead of print June 4, 2007]. J Neurol Sci 2007; 261:127–133. doi:10.1016/j.jns.2007.04.045