Tendinopathy
Original Editors - Evelyne Bahire as part of the Vrije Universiteit Brussel's Evidence-based Practice project
Top Contributors - Admin, Evelyne Bahire, Matthias Verlinden, Shaimaa Eldib, Jochem Vermeire, Matt Huey, Rucha Gadgil, Wanda van Niekerk, Amanda Ager, Lucinda hampton, Evan Thomas, WikiSysop, Simisola Ajeyalemi, Kim Jackson, Laura Mertens and Uchechukwu Chukwuemeka
Definition/Description[edit | edit source]
Tendinopathy ( pain and dysfunction in tendon) [1]defined as a failed healing response of the tendon, with haphazard proliferation of tenocytes, intracellular abnormalities in tenocytes, disruption of collagen fibers, and a subsequent increase in noncollagenous matrix.[2][3][4] The term tendinopathy is a generic descriptor of the clinical conditions ( both pain and pathological characteristics) associated with overuse in and around tendons.[5]Also, it is characterised with pain , reduced function and exercise intolerance. [6]
Clinically Relevant Anatomy[edit | edit source]
Healthy tendons are white in color and have a fibroelastic structure. Within the extracellular network , tenoblasts and tenocytes constitute about 90% to 95% of the cellular elements of tendons.[7] The remaining 5% to 10% of the cellular elements of tendons consists of chondrocytes at the bone attachment and insertion sites, synovial cells of the tendon sheath, and vascular cells, including capillary endothelial cells and smooth muscle cells of arterioles.
The oxygen consumption of tendons and ligaments is 7.5 times lower than that of skeletal muscles[8]. The low metabolic rate and well-developed anaerobic energy-generation capacity are essential to carry loads and maintain tension for long periods, reducing the risk of ischemia and subsequent necrosis. However, a low metabolic rate results in slow healing after injury.[9]
Epidemiology /Etiology[edit | edit source]
Tendinopathic tendons have an increased rate of matrix remodeling, leading to a mechanically less stable tendon that is probably more susceptible to damage.[10] Histological studies of surgical specimens from patients with established tendinopathy consistently show either absent or minimal inflammation.[11][12][13] They generally also show hypercellularity, a loss of the tightly bundled collagen fiber appearance, an increase in proteoglycan content, and commonly neovascularization.[14][15] Inflammation seems to play a role only in the initiation, but not in the propagation and progression, of the disease process.[16] Failed healing and tendinopathic features have been associated with chronic overload, but the same histopathological characteristics also have been described when a tendon is unloaded: stress shielding seems to exert a deleterious effect.[17] Unloading a tendon induces cell and matrix changes similar to those seen in an overloaded state and decreases the mechanical integrity of the tendon.[18][19]
Characteristics/Clinical Presentation[edit | edit source]
Tendinopathy is usually seen in:
- Lateral Epicondylitis
- Medial Epicondylitis
- Patellar tendon
- Achilles Tendon [20]
- Rotator cuff
- Rotator Cuff Tendinopathy
The classic presentation is one of increasing pain at the site of the affected tendon, often with recognition that there has been an increase in activity. Usually the pain is load-related.
In very early tendinopathy, pain may be present at the beginning of an activity and then disappear during activity itself, only to reappear when cooling down if the activity is prolonged, or to be more severe on subsequent attempts to be active. The patient is usually capable to localize the pain rather clearly and the pain is described as ‘‘severe’’ or ‘‘sharp’’ during the early stages and sometimes as a ‘‘dull ache’’ once it has been present for some weeks.[21]
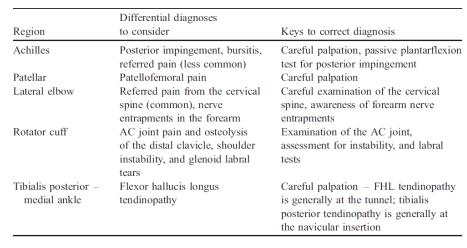
Differential Diagnosis[edit | edit source]
Specific differential diagnoses to consider when patients present with ‘tendinopathy’ at various anatomical regions.
Physical Examination[edit | edit source]
Examination includes inspection for muscle atrophy, asymmetry, swelling and erythema. Atrophy often is present with chronic conditions and is an important clue to the duration of the tendinopathy. Swelling, erythema, and asymmetry are commonly noted when examining pathologic tendons. Range-of-motion testing often is limited on the symptomatic side.[22]
Physical examination must include tests that load the tendon to reproduce pain and other loading tests that load alternative structures.
Medical Management[edit | edit source]
Use of injectable substances:
Corticosteroids improve short-term outcomes but are worse than no intervention or physiotherapy for intermediate- and long-term outcomes for some types of tendinopathy. Evidence is insufficient to evaluate effectiveness of other types of injection.[23]
Physical Therapy Management[edit | edit source]
Eccentric exercises
Eccentric exercises have been proposed to promote collagen fiber cross-link formation within the tendon, thereby facilitating tendon remodeling.[24]The basic principles in an eccentric loading regimen are unknown, although it has been speculated that forces generated during eccentric loading are of a greater magnitude than those in concentric exercises.[25] It is possible that eccentric exercises do not just exert a beneficial mechanical effect, but also act on pain mediators, decreasing their presence in tendinopathic tendons.[26]Excellent clinical results have been reported both in athletic and sedentary patients.[27] although these results were not reproduced by other study groups.[28] In general, the overall trend suggests a positive effect of eccentric exercises, with no reported adverse effects.[24]In one study, the combination of eccentric training and shock wave therapy produced success rates that were higher than those with eccentric loading alone or shock wave therapy alone.[29]
There is little consensus regarding which variables may influence the outcome of eccentric training, including whether training should be painful, home- vs clinic-based training, the speed of the exercise, the duration of eccentric training and the method of progression. Three basic principles in an eccentric loading regime have been proposed [24]:
- Length of tendon: if the tendon is pre-stretched, its resting length is increased, and there will be less strain on that tendon during movement.
- Load: by progressively increasing the load exerted on the tendon, there should be a resultant increase in inherent strength of the tendon.
- Speed: by increasing the speed of contraction, a greater force will be developed.
Yet more research is needed to confirm these modalities.
Extracorporeal Shockwave Therapy
Extracorporeal shock wave therapy (ESWT or SW) is a mechanotherapy (high-energy electromagnetic waves) that has recently become popularized for the management of musculoskeletal disorders. It is most frequently applied for the treatment of tendinopathies.[30]
ESWT is of interest to clinicians for two reasons. Firstly, it is said to stimulate the metabolic activity of the targeted cells, to promote tissue healing[31] and secondly it is hypothesized to have an influence on the localized nociceptors, resulting in a pain management effect.
ESWT is defined as a "...sequence of single sonic pulses characterized by high peak pressure – 100 MP, a fast onset of pressure (<10 ns), and short duration (10 µs). ESWT is conveyed by an appropriate generator to a specific target area with an energy density in the range of 0.003–0.890 mJ/mm2""[32]
The transduction of an ESWT acoustic shock wave signal is converted into a biological signal which results in cell proliferation and/or differentiation via a mechano-transduction process.[33] Most research regarding ESWT has focused on better understanding the mechanisms which results in a mechanosensitive feedback between the acoustic impulses and the specifically stimulated physiological cells. The stimulated cells are said to be the extracellular matrix (ECM)-binding proteins and the nucleus via the cytoskeleton.[34] The mechanisms that enable tissues to recognize and convert the intensity, frequency, amplitude and duration of an acoustic signal into a biological reaction are still not fully understood.[30]
The effects of ESWT for pain management is also not fully understood. The mechanical stimulation of ESWT is said to occur with the primary afferent nociceptive C-fibers, and that both activation and sensitization can occur among the localized tissues.[35]
The rationale behind the clinical use of extracorporeal shock wave therapy remains the stimulation of soft-tissue healing and the inhibition of the pain receptors (nociceptors). There is no consensus on the use of repetitive low-energy extracorporeal shock wave therapy, which does not require local anesthesia, versus the use of high-energy extracorporeal shock wave therapy, which requires local or regional anesthesia.[36]
Low-level laser therapy (LLLT)
There is no consensus about the use of low-level laser treatment for tendinopathies. And a number of question remain unanswered, like LLLT’s role when used in combination with other interventions, and especially exercises, in the remodeling phase of the tendon repair.[37]
Iontophoresis and phonophoresis
Iontophoresis and phonophoresis involve using ionizing current or ultrasound to deliver medications locally. Corticosteroids and NSAIDS are commonly used with these modalities.
Both are widely used and anecdotally effective, but well-designed RCTs are lacking to permit reliable recommendations.[38]
Friction massage
Friction is defined as “an accurately delivered penetrating pressure applied through fingertips”. But there is currently little evidence available to support the use of it in the treatment of tendinopathy. A Cochrane review evaluating deep friction massage found no benefit with deep friction massage over other treatments.[39]
Ultrasound
Therapeutic ultrasound is commonly used in the treatment of tendinopathy . Despite this, there is little clinical research documenting the efficacy of ultrasound in treating tendinopathy or promoting tendon healing.
A majority of in vivo studies have documented the effectiveness of ultrasound treatment.
But in the era of evidence-based practice, further studies, especially randomized control trials, are essential in elucidating the efficacy of therapeutic ultrasound in promoting tendon healing and treating tendinopathy.[40]
The only areas where ultrasound showed slight promise was in the treatment of lateral epicondylitis and calcific tendinopahty of the supraspinatus, some controlled trials and a systemic review demonstrated a benefit of using therapeutic ultrasound.
Hyperthermia
Early data on hyperthermia are encouraging, but remain preliminary. Only two randomized clinical trials (from a single institution) have been published evaluating hyperthermia compared to therapeutic ultrasound in the treatment of tendinopathy. These trials report improvements in pain and patient satisfaction in the hyperthermia group compared to the ultrasound group.[41][42]
Resources[edit | edit source]
 |
Achilles Tendinopathy Toolkit
The Achilles Tendinopathy Toolkit is a comprehensive evidence based resource to assist practitioners in clinical decision making for Achilles Tendinopathy. |
Clinical Bottom Line[edit | edit source]
In general, it would be reasonable to treat a patient with tendinopathy with physical therapy involving a program of eccentric exercises, to be performed for twelve weeks. If the condition does not respond to this intervention, shock wave therapy or a nitric oxide patch[43] might be considered, although data on their efficacy are limited. The use of operative treatment should be discussed with the patient after at least three to six months of nonoperative management. Moreover, patients should understand that symptoms may recur with either conservative or operative approaches.[2]
References[edit | edit source]
- ↑ Cardoso TB, Pizzari T, Kinsella R, Hope D, Cook JL. Current trends in tendinopathy management. Best Practice & Research Clinical Rheumatology. 2019 Feb 1;33(1):122-40.
- ↑ 2.0 2.1 Maffulli et al. Novel Approaches for the Management of Tendinopathy. J Bone Joint Surg Am. 2010;92:2604-2613. doi:10.2106/JBJS.I.01744
- ↑ Maffulli N, Longo UG, Maffulli GD, Rabitti C, Khanna A, Denaro V. Marked pathological changes proximal and distal to the site of rupture in acute Achilles tendon ruptures. Knee Surg Sports Traumatol Arthrosc. 2010.
- ↑ Maffulli N, Longo UG, Franceschi F, Rabitti C, Denaro V. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008; 466:1605-11.
- ↑ Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-3.
- ↑ Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GA, McInnes IB, Rodeo SA. Tendinopathy. Nature reviews Disease primers. 2021 Jan 7;7(1):1.
- ↑ Kannus P, Jozsa L, Jarvinnen M. Basic science of tendons. Principles and practice of orthopaedic sports medicine. Philadelphia: Lippincott Williams and Wilkins; 2000. p 21-37.
- ↑ Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18(10):1208-46. doi: 10.1089/ars.2011.4498.
- ↑ Lorenz D, Reiman M. The role and implementation of eccentric training in athletic rehabilitation: tendinopathy, hamstring strains, and acl reconstruction. Int J Sports Phys Ther. 2011 Mar;6(1):27-44. PMID: 21655455; PMCID: PMC3105370.
- ↑ Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108:670-5.
- ↑ Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Forriol F, Denaro V. Light microscopic histology of supraspinatus tendon ruptures. Knee Surg Sports Traumatol Arthrosc. 2007;15:1390-4.
- ↑ Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V. Characteristics at haematoxylin and eosin staining of ruptures of the long head of the biceps tendon. Br J Sports Med. 2009;43:603-7.
- ↑ Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36:533-8.
- ↑ Longo UG, Ronga M, Maffulli N. Acute ruptures of the Achilles tendon. Sports Med Arthrosc. 2009;17:127-38.
- ↑ Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112-26.
- ↑ Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37:1855-67.
- ↑ Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012 Apr 1;1(4):134-7. PMID: 23738261; PMCID: PMC3666485.
- ↑ Lewis JS. Rotator cuff tendinopathy: a model for the continuum of pathology and related management. Br J Sports Med. 2010 Jun 11.
- ↑ Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409-16
- ↑ Longo UG, Ronga M, Maffulli N. Achilles tendinopathy. Sports medicine and arthroscopy review. 2018 Mar 1;26(1):16-30.
- ↑ Fearon A, Neeman T, Smith P, Scarvell J, Cook J. Pain, not structural impairments may explain activity limitations in people with gluteal tendinopathy or hip osteoarthritis: A cross sectional study. Gait Posture. 2017 Feb;52:237-243. doi: 10.1016/j.gaitpost.2016.12.005.
- ↑ John J. Wilson, M.D., And Thomas M. Best, M.D., Ph.D. Common Overuse Tendon Problems: A Review and Recommendations for Treatment. Am Fam Physician. 2005;72(5):811-818.
- ↑ Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376: 1751-67.
- ↑ 24.0 24.1 24.2 Maffulli N, Longo UG. How do eccentric exercises work in tendinopathy? Rheumatology (Oxford). 2008;47:1444-5.
- ↑ Rees JD, Lichtwark GA, Wolman RL, Wilson AM. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology (Oxford). 2008;47:1493-7.
- ↑ Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2004;12:465-70.
- ↑ Roos EM, Engstr¨om M, Lagerquist A, S¨oderberg B. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy— a randomized trial with 1-year follow-up. Scand J Med Sci Sports. 2004;14:286-95
- ↑ Maffulli N, Longo UG, Loppini M, Denaro V. Current treatment options for tendinopathy. Expert Opin Pharmacother. 2010;11:2177-86.
- ↑ Rompe JD, Furia J, Maffulli N. Eccentric loading versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: a randomized controlled trial. Am J Sports Med. 2009;37:463-70.
- ↑ 30.0 30.1 Romeo, P., Lavanga, V., Pagani, D., & Sansone, V. (2013). Extracorporeal Shock Wave Therapy in Musculoskeletal Disorders: A Review Med Princ Pract. 2013 Dec; 23(1): 7–13.doi: 10.1159/000355472
- ↑ Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007;35:374-83
- ↑ Moon, S.W., Kim, J.H., Jung, M.J., Son, S., Lee, J.H., et al. The effect of extracorporeal shock wave therapy on lower limb spasticity in subacute stroke patients. Ann Rehabil Med, 2013,37:461–470.
- ↑ Shrivastava, S.K., & Kailash, K. Shock wave treatment in medicine. J Biosci, 2005, 30:269–275.
- ↑ Goodman M, Lumpkin E, Ricci A, et al. Molecules and mechanisms of mechanotransduction. J Neurosci. 2004;24:9220–9222.
- ↑ Klonschinski, T., Ament, S.J., Schlereth, T., Rompe, J.D., Birklein, F. Application of local anesthesia inhibits effects of low-energy extracorporeal shock wave treatment (ESWT) on nociceptors. Pain Med. 2011, 12(10):1532-7. doi: 10.1111/j.1526-4637.2011.01229.x.
- ↑ Rompe JD, Maffulli N. Repetitive shock wave therapy for lateral elbow tendinopathy (tennis elbow): a systematic and qualitative analysis. Br Med Bull. 2007; 83:355-78.
- ↑ Bjordal J, Couppe C, Ljunggren A. Low level laser therapy for tendinopathy, Evidence of a dose-response pattern. Physical Therapy Reviews 2001; 6: 91-99
- ↑ Wilson J, Best T. Common Overuse Tendon Problems: A Review and Recommendations for Treatment. Am Fam Physician. 2005;72(5):811-818.
- ↑ Brosseau L, Casimiro L, Milne S, Robinson V, Shea B, Tugwell P, Wells G. Deep transverse friction massage for treating tendinitis. Cochrane Database Syst Rev. 2002: 4: CD003528.
- ↑ Tsai W-C, Tang SF-T, Liang F-C: Effect of therapeutic ultrasound on tendons. Am J Phys Med Rehabil 2011;90:00-00
- ↑ Giombini A, Di Cesare A, Casciello G, Sorrenti D, Dragoni S, Gabriele P. Hyperthermia at 434 MHz in the treatment of overuse sport tendinopathies: a randomised controlled clinical trial. Int J Sports Med. 2002;23:207–211.
- ↑ Giombini A, Di Cesare A, Safran MR, Ciatti R, Maffulli N. Short-term effectiveness of hyperthermia for supraspinatus tendinopathy in athletes: a short-term randomized controlled study. Am J Sports Med. 2006;34:1247–1253.
- ↑ Murrell G. Using nitric oxide to treat tendinopathy. Br J Sports Med 2007;41:227–231. doi: 10.1136/bjsm.2006.034447