Posterior Fossa Syndrome
Introduction[edit | edit source]
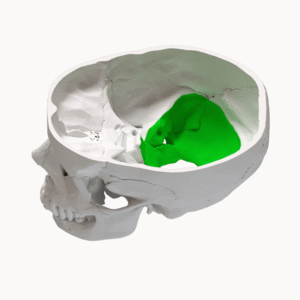
Posterior Fossa Syndrome (PFS), Cerebellar Mutism Syndrome, and Cerebellar Mutism are synonyms for a condition which can develop after surgery within the posterior fossa region of the cranium (Avula et al, 2015; Kupeli et al, 2011). The condition is characterized by a range of neurological problems such as: ataxia, mutism/dysarthria, hypotonia, emotional lability, and behavioral changes (Avula et al, 2015; Kupeli et al, 2011; Lanier & Abrams, 2017.) The recognition of a specific condition characterised by this group of symptoms has evolved over the past 44 years. Hirsch et al (1979) first notes the condition as a group of neuropsychological complications including speech disturbances in children who had underwent medulloblastoma surgery. Since then, differing presentations have been more widely studied, leading to a more accurate definition and diagnostic process. Previous iterations of diagnoses include but are not limited to pseudobulbar palsy, mutism without behavioral impairment, and cerebellar speech syndrome (Lanier & Abrams, 2017). More recent developments have suggested using the diagnosis "cerebellar cognitive affective syndrome", though most literature still uses PFS (Lanier & Abrams, 2017). The commonality between these diagnoses coupled with the ambiguity of the individual terms themselves sheds light on an increased recognition of PFS, variance in clinical presentations, and lack of understanding of the pathophysiology of PFS.
Etiology[edit | edit source]
There is no definitive understanding of the pathogenesis or pathophysiological process of PFS (Avula et al, 2015.) However, there are several theories which hypothesise processes which may lead to PFS.
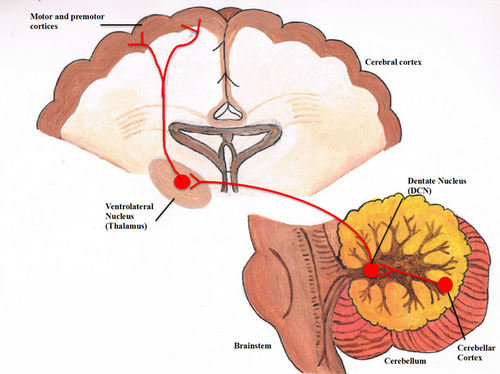
Dentato-Thalamo-Cortical
The DTC is a neural tract which connects the lateral cerebellum to the contralateral motor and non-motor areas of the cerebrum, such as the primary motor cortex, premotor cortex, and dorsal prefrontal cortex (Schulz et al, 2015). Interruption of the proximal segment of the DTC during surgery is what most authors believe to be the reason for the development of PFS. Therefore, the dentate nucleus and superior cerebellar peduncle are implicated in the development of PFS (Avula et al, 2015.) Reduced neural signaling from these structures due to disruption of the DTC is thought to result in cerebello-cerebral diaschisis, where lack of lack of stimulus from the cerebellum results in hypoperfusion and reduced metabolism within those connected cerebral structures (Avula et al, 2015; Soelva et al, 2013.) There are imaging studies which investigated cerebral perfusion in children with PFS which support this theory (Soelva et al, 2013.)
Cerebellar Vermis
The cerebellar vermis (CV) has been proposed to be involved in the development of PFS. This is a structure which divides the cerebellar hemispheres in half through the sagittal plane. Its function is not well understood but this structure has been associated with posture control and locomotion (Park et al, 2018). There are varied reports describing the degree to which the CV is potentially involved within the development of PFS (Avula et al, 2015.) Some authors report an association between a CV surgical approach and PFS incidence (Grill et al, 2004; Puget et al, 2009.) Other authors report no incidence of PFS after CV surgical approach (Wells et al, 2010.) Some authors note no difference in PFS incidence irrespective of surgical approach (Avula et al, 2015.)
Post-Operative Vasospasm
Some authors suggest that vasospasm of arteries supplying cerebellum and brainstem can cause a transient ischaemic attack, which could present as PFS (Avula et al, 2015). Vasospasm after neurosurgery is rare though recognised. It has also been recognised that surgery on sellar and middle cranial fossa tumours cause localized vasospasms, whereas surgery on posterior fossa tumours cause diffuse vasospasms with involvement of anterior and posterior cerebral circulation causing wide ranging neurological deficits (Avula et al, 2015; Jacob et al, 2011.) A review found that the average time between neurosurgery and the development of symptomatic vasospasm was 8 days (Alotaibi & Lanzino, 2013.) This does not fit with typical PFS presentation as most PFS symptoms occur within the first 2 days post-surgery. However, these are averages based on small sample sizes where no definitive conclusions can be drawn.
Post-Operative Edema
In rodent studies brain edema peaked at 24 hours post-op, remains increased for 3 days, then subsides by day 7 (Avula et al, 2015.) This is a convincing and intuitive theory of PFS pathogenesis. This fits in with our understanding of the chronology of the condition. Swelling within differing areas of the cerebellum may impact presentation and timeline of the condition. MRI studies on children who received posterior fossa surgery reveal that all participants showed diffusion abnormalities within the proximal efferent cerebellar pathway (Avula et al, 2015.) The edema located within this area was attributed to damage caused during surgery (Avula et al, 2015.)
Epidemiology[edit | edit source]
The is a geographically variable incidence of CNS tumours in children across the world, with rates ranging from 1.7-6.22 per 100,000 population, 58% of cases considered malignant (Formertin et al, 2023). Systematised by age groups, the incidence rates of CNS tumours for age groups 0–4 years (6.3 per 100,000) and 15–19 years (7.32 per 100,000) are higher than those observed in age groups 5–9 years (5.59 per 100,000) and 10–14 years (5.94 per 100,000) (Formentin et al, 2023). 8-40% of children who have undergone surgery within the posterior fossa will develop PFS (Avula et al, 2015; Kupeli et al, 2011; Thacker & Bouffet, 2021). Virtually all cases of posterior fossa syndrome will occur within the first week post surgery, with 50% of cases occurring within the first two days.
A recent study found that 34% (n=178) of children developed PFS after posterior fossa tumour resection. Symptomatically, 23% experienced complete mutism, 11% had diminished speech (Khan et al, 2021.) For those with mutism it took a median of 2.3 months for speech to return, and for those with diminished speech it took 0.7 months (Khan et al, 2021.) All children had severe ataxia. What is interesting is that it took a median 0.6 months longer for those with mutism to have their gait return to baseline, when compared to those with diminished speech (2.1 and 1.5 months respectively) (Khan et al, 2021.) 44.4% of children with mutism had been non-ambulatory at a 1 year follow up. High ataxia score and delayed speech recovery were associated with delayed gait return (P<0.0001).
Clinically Relevant Anatomy[edit | edit source]
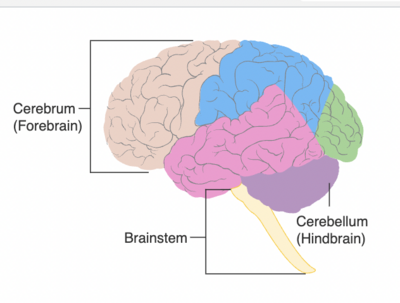
Cerebellum means “little brain” in Latin. The cerebellum is part of the hindbrain, inferior to the occipital and temporal lobes, located within the posterior cranial fossa. It is separated from these lobes by a tough layer of dura matter. The cerebellum lies at the same level as the pons, which is part of the brainstem. The cerebellum and pons are separated by the fourth ventricle.
The cerebellum consists of two hemispheres which are separated by the vermis. Each hemisphere consists of three separate anatomical lobes: The Anterior Lobe, The Posterior Lobe, and the The Flucculonodular Lobe
The cerebellum can also be divided into functional zones:
- Cerebrocerebellum: The largest division, formed by the lateral hemispheres. It is involved in planning movements and motor learning. This area also regulates coordination of muscle activation and is important in visually guided movements.
- Spinocerebellum: Comprised of the vermis and intermediate zone of the cerebellar hemispheres. It is involved in regulating body movements by allowing for error correction. It also receives proprioceptive information.
- Vestibulocerebellum: Connections to vestibular nuclei which use information about head movement to influence eye movement. This lobe is also associated with control of balance and tone. This is the oldest part of the brain in evolutionary terms.
Risk Factors[edit | edit source]
Tumour Type: Medulloblastomas are the most common type of malignant CNS tumours in children, making up 20-40% of all tumours (Kupeli et al, 2011). These tumours often warrant surgical intervention as part of the treatment plan, increasing risk of developing PFS.
Tumour Location: 54-70% of CNS tumours in children are located in the posterior fossa (Kupeli et al, 2011). In adults this number reduces to only 15-20%. Mid line tumours are more common in those who develop PFS. With one study reporting that 90% of patients who developed PFS had a mid line tumour (Kupeli et al, 2011).
Age: Due to posterior fossa tumours, and subsequently posterior fossa surgery, being potentially over 3x more common in paediatrics, it follows that being younger in age is a risk factor for developing PFS.
Surgical: Complications during surgery may possibly lead to PFS. Surgery to the cerebellar vermis an identified potential risk factor. However, the literature around this is inconclusive (Avula et al, 2015).
Symptoms/Conditions which warrant surgery: hydrocephalus and brainstem compression (Avula et al, 2015.)
It is unclear whether these risk factors are causal in the development of PFS. Hydrocephalus for example may be co-occurring in posterior fossa tumours due to the tumour causing changes in CSF flow. Medulloblastoma is only a risk factor as far as surgical intervention for the tumour is an option. If surgical intervention was not carried out, then the incidence of PFS in that population may decline. The main risk factor seems to be surgical intervention its self.
Clinical Presentation[edit | edit source]
PFS typically develops 1-day post-surgery and can last 1-day to several months (Kupeli et al, 2011.) There is no consistent presentation of symptoms when developing PFS. Instead, a patient may present with one of, or any number of, these symptoms. Furthermore, symptoms do not have to develop simultaneously. The main symptoms include:
Mutism
Presentation varies widely across a wide population base. The term ‘mutism’ has been attributed to various iterations of names of this condition since its discovery. This is due to one symptom of PFS being mutism, or more widely, dysarthria. Total loss of speech is not always the case, weakness throughout the muscles involved in speech can also be a symptom.
Ataxia
This is as a reduction in balance and coordination. This can manifest as unsteady gait or balance. The severity of these deficits can vary. One may be able to sit down and maintain their trunk balance with ease, though may find walking extremely difficult. Hypotonia may also develop, further effecting balance and coordination.
Emotional Lability
This is described as sudden changes in mood, where the emotions experienced are often very strong (Lanier & Abrams, 2016.) For example, moving quickly between inconsolable crying, hysteric laughter, distressed, distracted, and apathetic to name a few.
Behavioral Symptoms
Descriptions of this are more vague within the literature but is generally defined as someone who may be withdrawn, regressing in personality, lacking spontaneous movement, and restless (Lanier & Abrams, 2016.) Though any unexpected changes in behavior post-op may be taken to represent PFS.
Management/Interventions[edit | edit source]
Physiotherapy
Due to the cerebellum being the centre for balance and coordination, it is likely that our focus will be improving these areas. It is likely due to the location of a tumour that there was mass effect on the cerebellum and brainstem, meaning that the patient’s balance/coordination was probably affected pre-operatively. This gives the physiotherapy team, the patient, and the family a good opportunity to begin to receive physiotherapy input before surgery and familiarise themselves with the type of exercise we may do post-surgery.
Ataxia: The prevention of falls is a major component to consider when treating patients with ataxia. Due to this, patients require a very thorough assessment of their balance and fall risk to determine their mobility status. Immediate action may have to be taken if it is deemed to unsafe for a personal to continue mobilizing without appropriate aids such as:
- Standing Frame
- Wheelchair
- Gutter Frame
- Assistance of others
- Close support
- Supervision
Once a detailed assessment has been completed and appropriate aid/advice might have been given, a collaborative approach to goal setting and therapy planning can begin. Although providing physiotherapy for an ataxic patient can look different depending on their current situation, the main areas of focus are much the same. It’s likely that after having posterior fossa surgery that the patient’s mobility will be decreased, possibly leaving them in bed for most of the day during the early stages. During this it is important to assess the patient when providing treatment. Specifically, monitoring range of movement at areas in which there is a risk of contracture developing. Areas of particular concern are the ankle as when supine the feet tend to rest in a plantarflexed position, which would limit dorsiflexion range. Hamstrings are another area for concern, lying in bed provides no stretch to the hamstrings. Contracture within the hamstrings and calves can impact gait recovery. Gentle bed exercise and repositioning may be most appropriate during this stage. Later on, improving balance will likely be the main focus. This can be completed in sitting, standing, perching or any other position which adequately challenges the patient’s balance. Depending on position, reducing upper limb weight bearing will reduce base of support and challenge core stability in sitting, or standing balance if standing. Due to PFS affecting mainly children, this is best achieved through play, which is equally as fun as it is challenging. Play is a good opportunity for dynamic task practice which challenges balance.
Hypotonia: Encouraging movement within hypotonic muscles is the focus of interventions which address this issue. Children will often find it difficult to maintain focus on trying to move a limb or activate a muscle which is less effective or requires more effort than the contra-lateral side. This means that treatment sessions must be adapted to specifically target one limb more than the other. Facilitating reaching with the left upper limb by holding toys out to the left side of a child for example will encourage them to move that effected limb. If the patient is hypotonic for an extended length of time, resting splints may be appropriate.
Outcome Measures[edit | edit source]
Any outcomes measures which are age appropriate and focus on balance coordination are most appropriate during PFS assessment.
- Berg Balance Scale
- Tinetti
- Single leg stand
- Romberg
- Tandem Stand/Walk
- Heel/Shin test
- Finger/Nose chase
- Tone
- Modified Ashworth Score
References[edit | edit source]
- Alotaibi, N. M., & Lanzino, G. (2013). Cerebral vasospasm following tumor resection. Journal of neurointerventional surgery, 5(5), 413-418.
- Avula, S., Mallucci, C., Kumar, R., & Pizer, B. (2015). Posterior fossa syndrome following brain tumour resection: review of pathophysiology and a new hypothesis on its pathogenesis. Child's Nervous System, 31, 1859-1867.
- Formentin, C., Joaquim, A. F., & Ghizoni, E. (2023). Posterior fossa tumors in children: current insights. European Journal of Pediatrics, 182(11), 4833-4850.
- Grill, J., Viguier, D., Kieffer, V., Bulteau, C., Sainte-Rose, C., Hartmann, O., ... & Dellatolas, G. (2004). Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. Journal of Neurosurgery: Pediatrics, 101(2), 152-158.
- Khan, R. B., Patay, Z., Klimo Jr, P., Huang, J., Kumar, R., Boop, F. A., ... & Robinson, G. W. (2021). Clinical features, neurologic recovery, and risk factors of postoperative posterior fossa syndrome and delayed recovery: a prospective study. Neuro-oncology, 23(9), 1586-1596.
- Küpeli, S., Yalçın, B., Bilginer, B., Akalan, N., Haksal, P., & Büyükpamukçu, M. (2011). Posterior fossa syndrome after posterior fossa surgery in children with brain tumors. Pediatric Blood & Cancer, 56(2), 206-210.
- Lanier, J. C., & Abrams, A. N. (2017). Posterior fossa syndrome: Review of the behavioral and emotional aspects in pediatric cancer patients. Cancer, 123(4), 551-559.
- Puget, S., Boddaert, N., Viguier, D., Kieffer, V., Bulteau, C., Garnett, M., ... & Grill, J. (2009). Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer, 115(6), 1338-1347.
- Schulz, R., Wessel, M. J., Zimerman, M., Timmermann, J. E., Gerloff, C., & Hummel, F. C. (2015). White matter integrity of specific dentato-thalamo-cortical pathways is associated with learning gains in precise movement timing. Cerebral Cortex, 25(7), 1707-1714.
- Soelva, V., Hernáiz Driever, P., Abbushi, A., Rueckriegel, S., Bruhn, H., Eisner, W., & Thomale, U. W. (2013). Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Child's nervous system, 29, 597-607.
- Soelva, V., Hernáiz Driever, P., Abbushi, A., Rueckriegel, S., Bruhn, H., Eisner, W., & Thomale, U. W. (2013). Fronto-cerebellar fiber tractography in pediatric patients following posterior fossa tumor surgery. Child's nervous system, 29, 597-607.
- Thacker, N., & Bouffet, E. (2021). Posterior fossa syndrome—time to unmute the silence on cerebellar mutism. Neuro-oncology, 23(9), 1427-1428.
- Wells, E. M., Khademian, Z. P., Walsh, K. S., Vezina, G., Sposto, R., Keating, R. F., & Packer, R. J. (2010). Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. Journal of Neurosurgery: Pediatrics, 5(4), 329-334.. Journal of Neurosurgery: Pediatrics, 5(4), 329-334.