CREST Syndrome: Difference between revisions
Aya Alhindi (talk | contribs) No edit summary |
Aya Alhindi (talk | contribs) No edit summary |
||
| Line 179: | Line 179: | ||
==Physical Therapy Management== | ==Physical Therapy Management== | ||
Before beginning rehabilitation, the physiotherapist must do a thorough assessment of the patient in order to develop a suitable therapeutic programme especially upper limb functional and ROM assessment as the hands are the most commonly involved part of the body in scleroderma, with symptoms such as edema, Raynaud's phenomenon, sclerodactyly producing pain, and joint ROM reduction. The usual function of the hand is disrupted, and the patient has difficulty completing his ADL. | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Revision as of 00:18, 10 September 2023
This article or area is currently under construction and may only be partially complete. Please come back soon to see the finished work! (10/09/2023)
Original Editor - Aya Alhindi
Top Contributors - Aya Alhindi, Kim Jackson and Khloud Shreif
Description[edit | edit source]
CREST syndrome (also known as Cutaneous systemic sclerosis or limited scleroderma ) is an autoimmune disease that has been defined as a subtype of progressive systemic sclerosis (SSc) with limited skin involvement. [1]The word "CREST " is an acronym for the clinical features that are seen in a patient with this disease:
- Calcinosis- when calcium salts are deposited into the skin and subcutaneous tissue.[2]
- Raynaud's phenomenon.
- Esophageal dysmotility-which can cause difficulty in swallowing.
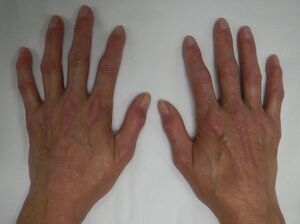
- Sclerodactyly- scleroderma in which the fingers become thin and shiny with sclerotic skin at the tip due to subcutaneous and intracutaneous calcinosis and diffused fibrosis of the collagen.[3]
- Telangiectasia-small widened blood vessels on the skin.[4]
Epidemiology[edit | edit source]
Wide variation in prevalence of systemic sclerosis was observed, with slightly higher estimates reported in North America (13.5–44.3 per 100,000 individuals) compared to Europe (7.2–33.9 per 100,000 individuals), which may be a true reflection of epidemiological variation or an artifact of clinical data analyses.[5] The apparent increase in both incidence and prevalence over the last 50 years is most likely due to improved classification, earlier diagnosis, and survival. CREST syndrome may account for 22-25% of all occurrences of systemic sclerosis, according to serum antibody investigations; however, epidemiologic research specifically looking at CREST syndrome are missing.[6] SSc diagnosis was reported to occur at the ages of 33.5-59.8 years in Europe and 46.1-49.1 years in North America, and to occur more commonly in women (female:male ratio of 3.8-11.5:1 in Europe and 4.6-15:1 in North America). Women have continuously greater prevalence and incidence rates, indicating a clinically significant difference in the occurrence of SSc across genders.[5]
Pathophysiology[edit | edit source]
The pathogenesis of SSc involves a classical triad of key mechanisms:[7]
- Endothelial dysfunction and apoptosis of endothelial cells.
- Uncontrolled activation of adaptive and innate immunity (notably including M1 inflammatory and M2 pro-fibrotic macrophages).
- Over-production of extracellular matrix (ECM) components by chronically activated myofibroblasts, resulting in the formation of a stiff and fibrotic extracellular matrix in numerous organs, interfering with their function.
Myofibroblasts are the primary contributors to ECM formation and fibrosis.In SSc, myofibroblasts are derived from a range of tissue-resident mesenchymal progenitor cell types, such as fibroblasts, pericytes, microvascular endothelial cells, and vascular preadipocytes]. In SSc, myofibroblasts undergo substantial epigenetic remodelling as well as metabolic changes such as increased glycolysis and altered NAD+ homeostasis.Furthermore, myofibroblasts in SSc exhibit apoptosis resistance as well as unregulated production of extracellular matrix (ECM) components such as collagens, tenascin C, and fibronectin. In turn, these released extracellular components can activate myofibroblasts either directly via innate immunological sensors such as TLR-4, or indirectly by mechano-sensing of increased matrix stiffness by integrins in an FAK dependent way. [7]
Regarding limited scleroderma, although the primary cause is unknown, it is reasonable to speculate that vascular endothelial cell abnormalities induce mononuclear infiltration, and that the resulting changes in TH1 and/or TH2 cell and cytokine balance result in abnormal fibroblast activity and increased collagen deposition.[8]
Diagnosis[edit | edit source]
In the absence of a diagnostic test proving the absence or presence of SSc, the diagnosis is based on a combination of clinical and laboratory findings. According to the latest classification scheme from 2013, SSc is confirmed by:[9]
Major Criteria:
- skin thickening of the fingers of both hands extending proximal to the metacarpophalangeal joints (MCP) is sufficient to classify a subject as having SSc.
- skin thickening sparing the fingers’ are classified as not having SSc.
Minor criteria:
- Skin thickening of the fingers -Puffy fingers and Sclerodactyly of the fingers (distal to the metacarpophalangeal joints but proximal to the proximal interphalangeal joints)
- Fingertip lesions -Digital tip ulcers and Fingertip pitting scars
- Telangiectasia
- Abnormal nailfold capillaries
- Pulmonary arterial hypertension and/or interstitial lung disease
- Raynaud’s phenomenon
- SSc-related autoantibodies:
- Anticentromere.
- Anti–topoisomerase I [anti–Scl-70].
- Anti–RNA polymerase III.
Etiology[edit | edit source]
While the exact cause of CREST syndrome is unknown, it is thought to be the result of a complex interplay of genetic, immunological, and environmental variables.
Calcinosis[edit | edit source]
Approximately 40% of patients with limited cutaneous SSc complicating from calcinosis (or dystrophic calcification) which is the accumulation of insoluble calcified material in the soft tissues, occurring in the presence of normal calcium and phosphate metabolism. The cause of how or why these crystals form in patients with SSc is not well understood.There is some poorly understood factors have all been proposed to contribute to calcinosis including:[10]
Chronic hypoxia
characterised by:
- Digital ulcers
- Loss of digital tip
- Abnormal capillary drop-outs seen by nailfold Capilloroscopy
Repetitive trauma
- Based on common locations of these deposits such as the fingertips and extensor surfaces of extremities.
Localised structural damage
Raynaud's phenomenon[edit | edit source]
Raynaud’s phenomenon secondary to SSc occurs in 90% of patients and is often the earliest clinical manifestation to occur.[11]It is vasospastic disorder that characterized by frequent and sudden drops in blood flow to the fingertips, often in response to cold temperatures. Raynaud phenomenon is a symptom complex caused by impaired digital perfusion and can occur as a primary phenomenon or secondary to a wide range of underlying causes. [12]
The etiology of SSc-associated Raynaud phenomenon includes factors such as endothelial cell injury (possibly autoantibody mediated); an imbalance between vasoconstrictor and vasodilator molecules (such as endothelin 1 and nitric oxide, respectively); structural microvascular changes from progressive microangiopathy; and intravascular events that lead to luminal occlusive disease. [12]
Esophageal dysmotility[edit | edit source]
SSc and gastrointestinal manifestations have been proven to be associated , with more than 90% of SSc patients also having various types of gastrointestinal dysfunction, which is considered the third greatest cause of death in SSc patients . Esophageal micro-reflux, manifested as dysphagia and reflux heartburn, is the most common indication of gastrointestinal dysfunction involvement and, to some extent, exacerbates the existing interstitial lung diseases. Furthermore, Barrett's oesophagus, esophageal stenosis, and esophageal cancer induced by esophageal motility problems may worsen the prognosis of SSc patients.[13]The etiology of esophageal motility disorders in patients with SSc is still uncertain.Many functional tests show the presence of vascular damage, fibrosis, and inflammatory illness, however these three factors may not be a complete picture and may lead to undetected etiology. Previously, some studies reporting the related pathological observations revealed esophageal muscle atrophy without evidence of vascular damage, fibrosis, or inflammatory infiltration. However, the exact mechanism of esophageal muscle atrophy is not known. [13]
Sclerodactyly[edit | edit source]
Sclerodactyly develops from a perivascular inflammatory infiltration in the dermis.[14]Although the cause of this inflammatory process is still unknown,it is believed that mucopolysaccharide, glycoprotein, and collagen (types I and III) deposition in the dermis causes the edematous phase of skin involvement.As collagen deposition progresses, the dermis becomes sclerotic rather than edematous. Meanwhile, in small arteries, a similar mechanism happens and in the intima, mucinous deposition occurs. [15]The adventitia is initially invaded by inflammatory cells before becoming fibrotic. This process causes artery narrowing, followed by arterial collapse or thrombosis.As a result the tissue becomes ischemic.Fibrosis typically disappears years after the start of skin changes, leaving atrophic skin behind.[15]
Telangiectasia[edit | edit source]
Prominent and numerous telangiectasia are a common clinical symptoms of scleroderma.The etiology of telangiectasia in general, and CREST in particular, is unknown. Models have not explained the preference for hands, face, and mucosa, as well as their proclivity to enlarge in diameter with time. Venous hypertension as a cause appears implausible given the rarity of high venous pressures in the foot. While both Raynaud's phenomenon and telangiectasia are present in CREST, telangiectasia is not present in primary Raynaud's disease, implying that telangiectasia is not caused by recurrent vasospasm or vasoconstriction. Telangiectasia is also widespread on the face, which is unaffected by Raynaud's phenomenon.[16]
Medical Management[edit | edit source]
Because a scleroderma diagnosis will affect a patient's physical and psychological well-being, a comprehensive approach to care should be taken. An assessment of organ involvement is required, as well as patient education about the clinical course, patient and family support, and treatment based on disease severity and organ involvement. A rheumatologist should be consulted.[17]
Organ-specific treatment can have a direct effect on outcomes such as mortality, disease progression, and quality of life issues.[18]
| Problem | Treatment |
|---|---|
| Arthralgias | NSAIDs, methotrexate, Cox-2 inhibitors |
| End-stage lung disease | Lung transplantation |
| Esophageal reflux | Proton pump inhibitors, metoclopramide |
| Intestinal dysmotility | Antibiotics, if malabsorption present; prokinetics |
| Pulmonary hypertension | Calcium channel blockers, epoprostenol |
| Lung inflammation | Cyclophosphamide |
| Inflammatory myositis | Methotrexate, prednisone |
| Raynaud’s phenomenon | Calcium channel blockers, cold avoidance,
angiotensin receptor blockers, nitroglycerin, digital sympathectomy |
| Renal crisis | Aggressive blood pressure control, including
angiotensin 1- converting enzyme inhibitors; dialysis |
Raynaud’s phenomenon management[edit | edit source]
In approximately 50% of the patients Raynaud’s phenomenon is often very severe and can progresses to digital ulceration (DU).[19]First, Raynaud's phenomenon is treated, and then digital ulceration is treated. The two are purposely combined since optimising Raynaud's phenomenon treatment is a critical initial step in the prevention and treatment of SSc-related digital ulcers. Lifestyle changes (including patient education) and vasoactive medication treatments are recommended for the best management of SSc-related Raynaud's phenomenon. [20]
Pharmacological management of digital ulceration (DU) can include:[20]
- Vasoactive therapies.
- Other pharmacological therapies (excluding procedural treatments) including antibiotic therapy and analgesia.
- Procedural pharmacological therapies.
- Treatment of the acute DU (this can be a medical emergency).
Calcinosis management[edit | edit source]
Calcinosis cutis is difficult to treat pharmacologically, and a range of medications, including bisphosphonates, intralesional corticosteroids, aluminium hydroxide, warfarin, and diltiazem, have been attempted with poor effectiveness. The current available therapeutic option is local excision of uncomfortable or ulcerated nodules, but local recurrence is prevalent. [21]
Esophageal dysmotility management[edit | edit source]
In those with systemic/localized scleroderma (SSc) or limited scleroderma, the gastrointestinal tract (GI) is the second most affected organ system. SSc can impact any portion of the GI tract, from the mouth cavity to the anorectum. [22]
| GI part | Treatment |
|---|---|
| Oral cavity | rehabilitation via orofacial exercises and the administration of cevimeline, pilocarpine, muscarinic agonists, and artificial saliva. |
| Esophagus | Lifestyle management including head elevation at night, excluding triggering foods/substance abuse, and consuming small/frequent meals during the day.
Proton pump inhibitors (PPIs). Endoscopic dilatationEndoscopic ablation or resection of dysplastic epithelium using photochemical, thermal, or radio ablation energy is recommended in Barrett’s esophagus. |
| Stomach | Dietary modifications (low-fat/fiber-based diet and vitamin supplementation) are the first line for gastroparesis |
| Small intestine | Antibiotics such as ciprofloxacin, norfloxacin, amoxicillin, tetracyclines (doxycycline), metronidazole, and trimethoprim-sulfamethoxazole are effective against small intestinal bacterial overgrowth (SIBO). |
| Colon and anorectal | stimulant laxatives and stool softeners for constipation management. |
Cutaneous manifestations management[edit | edit source]
Current medicines are restricted and inadequate in treating scleroderma's cutaneous symptoms. Autologous fat transfer (AFT) is a surgical procedure for face rejuvenation that has been used for many decades. Adipose stem cells (ASCs) found in fat grafts have also showed promise in terms of anti-inflammatory and regenerative characteristics. AFT has recently been repurposed to treat systemic sclerosis and localised scleroderma skin symptoms. AFT appears to enhance mouth and hand functions, Raynaud's symptoms, and digital ulcerations in scleroderma patients, according to research. AFT is a safe operation with little postoperative problems, making it a prospective option for scleroderma treatment. More research is needed to properly characterise the impact of fat grafts on the recipient site and to define fat transfer criteria in fibrotic skin diseases.[23]
Physical Therapy Management[edit | edit source]
Before beginning rehabilitation, the physiotherapist must do a thorough assessment of the patient in order to develop a suitable therapeutic programme especially upper limb functional and ROM assessment as the hands are the most commonly involved part of the body in scleroderma, with symptoms such as edema, Raynaud's phenomenon, sclerodactyly producing pain, and joint ROM reduction. The usual function of the hand is disrupted, and the patient has difficulty completing his ADL.
References[edit | edit source]
- ↑ Meyer O. CREST syndrome. Ann Med Interne (Paris) [Internet]. 2002;153(3):183–8. Available from: https://europepmc.org/article/med/12218901
- ↑ Le C, Bedocs PM. Calcinosis Cutis. StatPearls Publishing; 2023.
- ↑ Nelson FRT, Blauvelt CT. The Hand and wrist. In: Nelson FRT, Blauvelt CT, editors. A Manual of Orthopaedic Terminology. Elsevier; 2015. p. 307–41.
- ↑ Telangiectasia (Spider Veins) [Internet]. Pennmedicine.org. [cited 2023 Sep 8]. Available from: https://www.pennmedicine.org/for-patients-and-visitors/patient-information/conditions-treated-a-to-z/telangiectasia-spider-veins
- ↑ 5.0 5.1 Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol [Internet]. 2019;11:257–73. Available from: http://dx.doi.org/10.2147/clep.s191418
- ↑ Wangkaew S, Euathrongchit J, Wattanawittawas P, Kasitanon N, Louthrenoo W. Incidence and predictors of interstitial lung disease (ILD) in Thai patients with early systemic sclerosis: Inception cohort study. Mod Rheumatol [Internet]. 2016;26(4):588–93. Available from: https://academic.oup.com/mr/article-pdf/26/4/588/39351804/mr0588.pdf
- ↑ 7.0 7.1 Lescoat A, Varga J, Matucci-Cerinic M, Khanna D. New promising drugs for the treatment of systemic sclerosis: pathogenic considerations, enhanced classifications, and personalized medicine. Expert Opin Investig Drugs [Internet]. 2021;30(6):635–52. Available from: https://pubmed.ncbi.nlm.nih.gov/33909517/
- ↑ De Martinis M, Ciccarelli F, Sirufo MM, Ginaldi L. An overview of environmental risk factors in systemic sclerosis. Expert Rev Clin Immunol [Internet]. 2016;12(4):465–78. Available from: https://pubmed.ncbi.nlm.nih.gov/26610037/
- ↑ van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis and rheumatism [Internet]. 2013 [cited 2023 Sep 8];65(11). Available from: https://pubmed.ncbi.nlm.nih.gov/24122180/
- ↑ Hsu V, Varga J, Schlesinger N. Calcinosis in scleroderma made crystal clear. Curr Opin Rheumatol [Internet]. 2019;31(6):589–94. Available from: https://journals.lww.com/co-rheumatology/abstract/2019/11000/calcinosis_in_scleroderma_made_crystal_clear.7.aspx
- ↑ Chang SH, Jun JB, Lee YJ, Kang TY, Moon KW, Ju JH, et al. A clinical comparison of an endothelin receptor antagonist and phosphodiesterase type 5 inhibitors for treating digital ulcers of systemic sclerosis. Rheumatology (Oxford) [Internet]. 2021;60(12):5814–9. Available from: https://academic.oup.com/rheumatology/article-pdf/60/12/5814/41820430/keab147.pdf
- ↑ 12.0 12.1 1. Hughes M, Allanore Y, Chung L, Pauling JD, Denton CP, Matucci-Cerinic M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol [Internet]. 2020;16(4):208–21. Available from: https://www.nature.com/articles/s41584-020-0386-4
- ↑ 13.0 13.1 Li B, Yan J, Pu J, Tang J, Xu S, Wang X. Esophageal dysfunction in systemic sclerosis: An update. Rheumatol Ther [Internet]. 2021;8(4):1535–49. Available from: http://dx.doi.org/10.1007/s40744-021-00382-0
- ↑ Adigun R, Goyal A, Hariz A. Systemic Sclerosis. StatPearls Publishing; 2022.
- ↑ 15.0 15.1 Foti R, De Pasquale R, Dal Bosco Y, Visalli E, Amato G, Gangemi P, et al. Clinical and histopathological features of Scleroderma-like disorders: An update. Medicina (Kaunas) [Internet]. 2021;57(11):1275. Available from: https://www.mdpi.com/1648-9144/57/11/1275
- ↑ Halachmi S, Gabari O, Cohen S, Koren R, Amitai DB, Lapidoth M. Telangiectasis in CREST syndrome and systemic sclerosis: correlation of clinical and pathological features with response to pulsed dye laser treatment. Lasers Med Sci [Internet]. 2014;29(1):137–40. Available from: http://dx.doi.org/10.1007/s10103-013-1298-1
- ↑ Joslin N. Early identification key to scleroderma treatment. Nurse Pract [Internet]. 2004;29(7):24–39; quiz 40–1. Available from: https://journals.lww.com/tnpj/abstract/2004/07000/early_identification_key_to_scleroderma_treatment.5.aspx
- ↑ Gelber AC, Wigley FM. Disease severity as a predictor of outcome in scleroderma. Lancet [Internet]. 2002;359(9303):277–9. Available from: http://www.thelancet.com/article/S0140673602075359/abstract
- ↑ Hughes M, Allanore Y, Chung L, Pauling JD, Denton CP, Matucci-Cerinic M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol [Internet]. 2020;16(4):208–21. Available from: https://pubmed.ncbi.nlm.nih.gov/32099191/
- ↑ 20.0 20.1 Herrick AL, Philobos M. Pharmacological management of digital ulcers in systemic sclerosis - what is new? Expert Opin Pharmacother [Internet]. 2023;24(10):1159–70. Available from: http://dx.doi.org/10.1080/14656566.2023.2213434
- ↑ Kempanna Y. P28 Recalcitrant exuberant digital calcinosis cutis in a patient of CREST syndrome - A case report. Rheumatol Adv Pract [Internet]. 2022;6(Supplement_1). Available from: https://academic.oup.com/rheumap/article-pdf/6/Supplement_1/rkac067.028/46053550/rkac067.028.pdf
- ↑ Nassar M, Ghernautan V, Nso N, Nyabera A, Castillo FC, Tu W, et al. Gastrointestinal involvement in systemic sclerosis: An updated review. Medicine (Baltimore) [Internet]. 2022;101(45):e31780. Available from: https://journals.lww.com/10.1097/MD.0000000000031780
- ↑ Kawakibi AR, Khouri AN, Cederna PS, Strong AL. Novel indications for autologous fat grafting in reconstruction: scleroderma. Plast Aesthet Res [Internet]. 2023;10(0):48. Available from: https://parjournal.net/article/view/6086