Female Athlete Triad: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 86: | Line 86: | ||
De Souza and colleagues (2020)<ref name=":15">De Souza MJ, Williams NI, Koltun KJ, Strock NC. Female Athlete Triad Coalition risk assessment tool is an evidenced-based tool that is reliable and well-described. Journal of Sports Sciences. 2020 May 2;38(9):996-9.</ref> addressed the differences in results found by Koltun and colleagues (2019)<ref name=":13" /> and Holtzman and colleagues (2019).<ref name=":14" /> De Souza and colleagues (2020)<ref name=":15" /> attributed them to the variability between the users, which stemmed from the lack of “explicit, well-defined risk factors and scoring instructions”<ref name=":15" /> for the RED-S tool. De Souza and colleagues (2020)<ref name=":15" /> recommend that the RED-S team implement revisions. However, they also noted that some limitations exist with the Triad Coalition tool, despite it having a more clearly defined scoring system for risk stratification.<ref name=":15" /> For ex. The Triad Coalition tool requires access to dual-energy X-ray absorptiometry and presents difficulty identifying low energy availability.<ref name=":15" /> In a recent article, Koltun and colleagues (2020)<ref>Koltun KJ, Williams NI, De Souza MJ. Female Athlete Triad Coalition Cumulative Risk Assessment Tool: Proposed alternative scoring strategies. Applied Physiology, Nutrition, and Metabolism. 2020 Jun 5(ja).</ref> proposed ways to address the limitations with the Triad Coalition tool,<ref name=":10" /> which included, amongst other things, substituting a delayed menarche for low bone mineral density (BMD and incorporating self-report questionnaires for low energy availability. | De Souza and colleagues (2020)<ref name=":15">De Souza MJ, Williams NI, Koltun KJ, Strock NC. Female Athlete Triad Coalition risk assessment tool is an evidenced-based tool that is reliable and well-described. Journal of Sports Sciences. 2020 May 2;38(9):996-9.</ref> addressed the differences in results found by Koltun and colleagues (2019)<ref name=":13" /> and Holtzman and colleagues (2019).<ref name=":14" /> De Souza and colleagues (2020)<ref name=":15" /> attributed them to the variability between the users, which stemmed from the lack of “explicit, well-defined risk factors and scoring instructions”<ref name=":15" /> for the RED-S tool. De Souza and colleagues (2020)<ref name=":15" /> recommend that the RED-S team implement revisions. However, they also noted that some limitations exist with the Triad Coalition tool, despite it having a more clearly defined scoring system for risk stratification.<ref name=":15" /> For ex. The Triad Coalition tool requires access to dual-energy X-ray absorptiometry and presents difficulty identifying low energy availability.<ref name=":15" /> In a recent article, Koltun and colleagues (2020)<ref>Koltun KJ, Williams NI, De Souza MJ. Female Athlete Triad Coalition Cumulative Risk Assessment Tool: Proposed alternative scoring strategies. Applied Physiology, Nutrition, and Metabolism. 2020 Jun 5(ja).</ref> proposed ways to address the limitations with the Triad Coalition tool,<ref name=":10" /> which included, amongst other things, substituting a delayed menarche for low bone mineral density (BMD and incorporating self-report questionnaires for low energy availability. | ||
== Diagnostic Tests/Lab Tests/Lab Values == | == Diagnostic Tests/Lab Tests/Lab Values == | ||
Revision as of 16:41, 18 June 2020
This article is currently under review and may not be up to date. Please come back soon to see the finished work! (18/06/2020)
Original Editors - Students from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - James Chad Cissell, Michelle Walsh, Ethan Adams, Aarti Sareen, Lucinda hampton, Wanda van Niekerk, Regan Haley, Kim Jackson, Elaine Lonnemann, Nicole Hills, 127.0.0.1, Oyemi Sillo, WikiSysop, Adam Vallely Farrell, Rishika Babburu and Claire Knott
Page Owner - Sadaf Tanveer as part of the One Page Project
Definition/Description[edit | edit source]
The Female Athlete Triad was originally defined as an interrelation of amenorrhea, osteoporosis, and disordered eating that would exist simultaneously.[1] More recently, it has been recognized that these 3 conditions exist on a spectrum and they have since been renamed menstrual dysfunction, low bone mineral density, and low energy availability with or without an eating disorder.[2] It is important to note that not all components of the Triad need to be be present to make the diagnosis; only one is needed.[2][3] Timely prevention, recognition, and treatment are important at delaying the progression as any one of the 3 Triad components places the individual at a higher risk of incurring all 3,[4][5] each component can have irreversible consequences,[2] and the components can increase in severity.
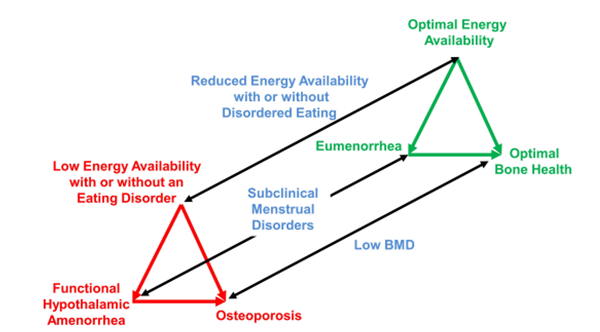
This is the Female Athlete Triad spectrum.[2] The black lines are representative of the spectrums of each of the 3 components and the red and green triangles show both of the extremes. The top right, green triangle, is desired and it is representative of a healthy athlete who has a good balance between energy intake and expenditure. Because of this, they have a normal menstruation cycle, and a bone mineral density that is above average for the athlete’s age. The bottom left, green triangle, is undesired and it is representative of an athlete who does not have an appropriate balance between energy intake and expenditure, which may be the result of restrictive dieting and/or clinical eating disorders. Because of this, they develop functional hypothalamic amenorrhea and osteoporosis (hormones, including estrogen, that promote the formation of bone are supressed).
Prevalence[edit | edit source]
The Female Athlete Triad occurs in girls and women, especially if they are highly competitive athletes. Development of Female Athlete Triad is also possible in those who are sedentary and recreationally active.[6][7] This just has been shown to occur at prevalence rates less than that of more competitive athletes.[8][9] Younger individuals are greatly impacted by the non-reversible, long-term consequences of this syndrome. In fact, a study on animals have showed that low energy availability can decrease growth and hinder sexual development.[10]
Unfortunately, due to inconsistencies and limitations in definition criteria and experimental design,[2][11] it is difficult to determine the prevalence of all three components of the Triad existing simultaneously. However, a lot of evidence exists looking at the prevalence of the individual components.[12]
A systematic review by Gibbs and colleagues (2013)[12] compiled available evidence and identified 9 studies looking at the prevalence of 3/3, 2/3, and 1/3 of the Triad conditions in exercising women. Of these 9 studies they included,[4][5][8][13][14][15][16][9][17] they found a prevalence of 0-15.9% for 3/3 conditions.[12] When it came to determining the prevalence of the combination of any 2 components they found the following: 1) menstrual dysfunction (MD) and low bone mineral density (BMD)- 0-7.5%, 2) MD and disordered eating (DE)- 2.7-50%, 3) low BMD and DE- 0.9 -3.2%, 4) MD and low energy availability (EA)- 17.5%, and 5) low BMD and low EA- 3.75%.[12] To look at the prevalence of individual components of the Triad in exercising women, Gibbs and colleagues (2013)[12] did more searches of the literature. They found that the prevalence ranged from 0-56% for primary amenorrhea, 1-60% for secondary amenorrhea, 0.9%-52.5% for oligomenorrhea, 7.1-89.2% for clinical and subclinical disordered eating, and 0-39.8% for low BMD (defined by z-score between -1.0 and -2.0).[12]
Gibbs and colleagues (2013)[12] also found literature that concluded an increased prevalence of the Triad components in lean sport athletes.[9][18][19][20][21][22][23][24][25] Lean sports are ones that emphasize endurance, aesthetic/physique, and/or antigravitation; non-lean sports are ones that are more technical, power-focused, and/or ball-related.[9] Torstveit and Sundgot-Borgen (2005)[9] found that the prevalence of DE and MD in lean sport athletes was nearly 2 times more likely than in non-lean sport athletes. This supports the notion that DE can lead to MD, which then leads to decreased BMD.[12]
Mechanisms[edit | edit source]
Low Energy Availability With or Without an Eating Disorder
Energy availability (EA) = dietary energy intake (calories in) – energy expended during exercise (calories out)
Where energy is measured in kilocalories (kcal).
Furthermore, a low EA can be due to decreased dietary energy intake and/or increased energy expended during exercise and, when EA is low, this leads to less energy available for body functions. Some athletes may participate in restrictive diets or use pills or laxatives.[4][18][23][26][27][28] Other athletes may have a diagnosis of an eating disorder including Anorexia Nervosa, Bulimia Nervosa, or Other Specified or Unspecified Feeding or Eating Disorders.
Low EA can lead to menstrual dysfunction and low BMD.
Menstrual Dysfunction
As shown in the Female Athlete Triad Spectrum, menstrual function will range from eumenorrhea to functional hypothalamic amenorrhea. Where eumenorrhea is a menstrual cycle occurring after 28 +/- 7 days and amenorrhea is absence of a menstrual cycle for more than 90 days.[2] Amenorrhea can be primary, meaning there is a delay in age of the first occurrence of menstruation (15 years or older or within 5 years of breast tissue development), or secondary, meaning it occurs after the individual has begun menstruation.[2] Oligomenorrhea is an irregularity somewhere in between the two extremes on the spectrum; it is defined as intervals of more than 35 days between menstrual cycles.[2]
Functional hypothalamic amenorrhea (FHA), which is most relevant in the Triad, can be a primary or secondary amenorrhea caused by low EA and it is classified into the following 3 categories: weight loss-related, stress-related, and exercise-related amenorrhea.[29] FHA affects gonadotropin-releasing hormone[30] and luteinizing hormone,[31] which leads to estrogen deficiency. The low EA, resulting in hypoestrogenism and other metabolic disturbances, can cause anovulation and infertility,[32] miscarriage or preterm birth,[33] low BMD and fractures,[34][35] coronary artery disease,[36] diabetes mellitus, anxiety,[37] and depression.[37]
Low Bone Mineral Density
Low BMD is classified as a z-score between -1.0 and -2.0 and osteoporosis is classified as a z-score of less than or equal to -2.0 along with the presence of a secondary risk factor such as low EA, hypoestrogenism, or a previous history of fractures.[2] Athletes engaging in weight-bearing sports have been shown to have a BMD that is 5-15% higher than those who are not engaging in any sports at all.[38][39][40][41] Furthermore, a z-score of -1.0 or less in athletes should lead to more tests regardless of what secondary risk factors are present at the time.[2] Approximately half of an individual’s peak bone mass (PBM) is formed during puberty[42] and, additionally, hormones and nutrition are thought to contribute 40-60%.[30] Low EA plays a big role in BMD—as evidenced by a randomized controlled trial that found bone formation in exercising women declined shortly after EA was reduced to less than 30kcal/kg.[43] A low EA can lead to estrogen deficiency, which plays a key role in bone formation. A loss in BMD may be irreversible so it is important to identify it as early as possible.
Characteristics/Clinical Presentation[edit | edit source]
- Weight loss
- Absent or irregular periods
- Fatigue
- Stress fractures
- Restrictive dieting
- Binge eating
- Induced vomiting
- Excessive exercise
Screening and Risk Factor Stratification[edit | edit source]
Screening for the Triad should occur for all female high school and college athletes[3] as part of the Pre-Participation Physical Evaluation (PPE),[44][45][46] at annual check-ups,[47][48][49] as well as during evaluation for the signs and symptoms related to the Triad (ex. stress fractures, menstrual dysfunction, etc).[2] Physiotherapists are often the first clinical encounter for many athletes. They have the ability to refer their patients onwards to undergo further investigations, which may require some advocating. Furthermore, having knowledge of the Triad and the ability to screen for it is compulsory in primary and secondary prevention. Screening questions can be incorporated into the subjective portion of physiotherapy assessments and treatments.[50]
Despite a number of validated tools that detect disordered eating, there wasn’t one that detected low energy availability (EA). Considering that evidence supports the role low EA plays in menstrual dysfunction and poor bone health, a screening tool to identify the Triad in its earlier stages would help to minimize the risk of long-term consequences. An observational study by Melin and colleagues (2014)[51] tested a questionnaire’s ability to detect athletes at risk of developing the Female Athlete Tried. These researchers created the LEAF-Q; a 25-item, self-report questionnaire with a sensitivity of 78% and a specificity of 90% for detecting low EA, menstrual dysfunction, and poor bone health.[51] Unfortunately this tool does not seem to be readily accessible online.
The 2014 Female Athlete Triad Coalition Consensus (Triad Coalition) Statement on Treatment and Return to Play of the Female Athlete Triad, by De Souza and colleagues (2014),[3] identified the following 9 questions that adolescent females should be asked as part of the PPE to screen for the Triad:[3]
- "Have you ever had a menstrual period?"
- "How old were you when you had your first menstrual period?"
- "When was your most recent menstrual period?"
- "How many periods have you had in the past 12 months?"
- "Are you presently taking any female hormones (oestrogen, progesterone, birth control pills)?"
- "Do you worry about your weight?"
- "Are you trying to or has anyone recommended that you gain or lose weight?"
- "Are you on a special diet or do you avoid certain types of foods or food groups?"
- "Have you ever had an eating disorder?"
- "Have you ever had a stress fracture?"
- "Have you ever been told you have low bone density (Osteopenia or Osteoporosis)?"
The De Souza and colleagues (2014)[3] also created a Cumulative Risk Assessment that classifies athletes into high risk, moderate risk, or low risk groups, which directly correlate with how much sport activity they should participate in. To find out the Cumulative Risk Score, risk factors are added together. Full clearance for participation requires 0-1 points, provisional/limited clearance requires 2-5 points, and restricted from training and competition requires 6 or more points.[3] Click here to access the article and the Cumulative Risk Assessment.
Around the same time, the International Olympic Committee (IOC) Consensus group[52] created an updated term for the Triad, called the Relatively Energy Deficiency in Sport (RED-S). Mountjoy and colleagues (2014)[52] believe RED-S is more suitable because it is not actually a triad, but a syndrome associated with numerous physiological impairments that affect health and performance. The RED-S Clinical Assessment tool can be readily accessed at the following link: RED-S Clinical Assessment Tool for the Evaluation of Athletes. With the tool, athletes can be classified into high risk, moderate risk, or low risk groups, which also correlate with how much sport activity they should participate in. Additionally, the RED-S risk assessment[52] also has a stepwise approach for determining an athlete’s readiness to return-to-play.
Koltun and colleagues (2019)[53] compared the Triad Coalition[3] and RED-S[52] tools and how they classified an athlete’s level of risk and recommendation for return-to-play using the same 166 individuals. Using the Triad Coalition tool, 25.3% of subjects were fully cleared, 62.0% were provisionally cleared, and 12.7% were restricted.[53] In comparison, using the RED-S tool, 71.7% were fully cleared, 18.7% were provisionally cleared, and 9.6% were restricted.[53] Furthermore, the both of the tools differ quite significantly in that the Triad Coalition tool was more conservative and the RED-S tool was more liberal.[53] Koltun and colleagues (2019)[53] conclude that this shouldn’t be concerning since the main differences seem to be in deciding whether an athlete should return-to-play fully or provisionally.
Holtzman and colleagues (2019)[54] performed another study comparing the 2 tools. The study involved 1000 athletes. The researchers found, using the Triad Coalition tool, 54.7% were moderate and 7.9% were high risk and, using the RED-S tool, 63.2% were moderate and 33% were high.[54] Holtzman and colleagues (2019)[54] conclude that the tools agree upon which athletes are at some level of risk, but not necessarily the exact level.
De Souza and colleagues (2020)[55] addressed the differences in results found by Koltun and colleagues (2019)[53] and Holtzman and colleagues (2019).[54] De Souza and colleagues (2020)[55] attributed them to the variability between the users, which stemmed from the lack of “explicit, well-defined risk factors and scoring instructions”[55] for the RED-S tool. De Souza and colleagues (2020)[55] recommend that the RED-S team implement revisions. However, they also noted that some limitations exist with the Triad Coalition tool, despite it having a more clearly defined scoring system for risk stratification.[55] For ex. The Triad Coalition tool requires access to dual-energy X-ray absorptiometry and presents difficulty identifying low energy availability.[55] In a recent article, Koltun and colleagues (2020)[56] proposed ways to address the limitations with the Triad Coalition tool,[3] which included, amongst other things, substituting a delayed menarche for low bone mineral density (BMD and incorporating self-report questionnaires for low energy availability.
Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
Athletes most commonly show signs of 1 or 2 components of Female Athlete Triad. If suspected due to pain, recurrent fractures, recurrent sprains, Low BMI (<85% expected weight), poor eating habits, or other, use the following questions to see if there is cause for concern.
Non-Modifiable:
- Female Gender
- Age 12-19
Modifiable:
- Early age sport specialization
- Low BMI (Z-score less than or equal to -1)
- Over-training
- Engaging in sports with endurance, aesthetic, and weight class components
- Severe Dieting
- Family Dysfunction
- Abuse
Energy Availability (EA)
- Energy Intake should be at least 45 kcal/kg of fat-free mass (FFM)[57]
- < 30kcal/kg FFM disrupts bone mineralization and menstruation[57]
- 5 days or more of < 30kcal/kg decreases luteinizing hormone availability in the body[57]
Triggers for disordered eating may include: Prolonged dieting, weight fluctuations, changes in coaching, injury, and social attention to weight.
Bone Mineral Density
In women, about 90% of bone formation should be complete by 18 years old[60], with bone density peaking between 20-30 years old. Poor Bone Mineral density is correlated with being <85% expected weight for height and age. A Z-score at or below -1.0 significantly increased risk for fractures and osteoporotic changes.
Long Distance Running is a highest risk sport for negatively impacting BMD
Use BMD Screenings to rule out other diagnoses such as celiacs disease[58]
Menstruation
Disorders in menstrual function can be as mild as anovulation and luteal dysfunction to Oligomenorrhea and Amenorrhea
Menstrual disturbances are common in all adolescents (~21%), but more common in adolescent athletes (~54%)[57]
- Anovulation - ovaries do not release an oocyte and ovulation doesn’t occur. (few overt symptoms)
- Luteal Deficiency - low concentration of blood progesterone and or Luteal phase lasting less than 11 days (few overt symptoms)
- Oligomenorrhea - menstrual cycles lasting longer than 35 days
- Primary Amenorrhea is the “absence of menarche by the age of 15 years.”[57]
- Secondary Amenorrhea is absence of menstruation for greater than or equal to 3 consecutive months after menarche
Systemic Involvement[edit | edit source]
- GI - low intake, atrophy, abnormal acid balance, and nutrient deficits
- Cutaneous - without appropriate nutrients, the skin becomes brittle and takes longer to heal
- Integumentary - lax weak ligaments from poor recovery time and nutrition.
- Musculoskeletal - inability to grow new muscle due to lack of resources and poor recovery time. Strength and endurance depletion from systemic catabolism.
- Cardiopulmonary and Circulatory - poor blood sugar, poor serum cholesterol and triglycerides, fragile balance of metabolites with decreased ability to compensate for imbalances due to poor storages. Reduced clotting factor. Increased incidence of endothelial dysfunction.
- Endocrine - increased production of stress hormones, decreased ability to balance homeostatic control due to poor systemic condition from prolonged stress and absent nutrients.
- Lymphatic and Immune - Immune system becomes fragile due to systemic stress.
- Neurological - decreased attention span. possibly poor balance.
- Reproductive System - Reproductive system shuts down (reduced blood flow, reduced metabolism, reduced hormone production) to maintain energy for vital functions.
Management[edit | edit source]
When approaching the Triad with an affected athlete it is important to recognize this may be a sensitive topic. Treatment requires a multidisciplinary approach.[2][61] The team may be comprised of various healthcare providers including a physician, registered dietitian, mental health practitioner, physiotherapist[2][62][63] (and/or athlete trainer or exercise physiologist), and coach.[50] While interventions can have both pharmacological and non-pharmacological components, non-pharmacological treatment methods are to be the initial course of action.[3] Pharmacological interventions should be considered if there is no improvement after a year of non-pharmacological intervention and/or the athlete has a relevant history of fractures.[3]
Pharmacological interventions: May include oral contraceptives, gonadal steroids (oestrogen, progesterone, and testosterone), other bone restorative medications, recombinant parathyroid hormone, antidepressants. However, as previously mentioned, pharmacological interventions should not be a first-line therapy. There is a lack of evidence to support them.[3]
Non-pharmacological interventions: Low energy availability (EA) is generally directly related to menstrual dysfunction and low bone mineral density (BMD) so it is addressed first and foremost.[2][52] Depending on the cause of low EA, the athlete should be referred to a sports dietitian for nutritional education and counselling. If there is suspicion of a clinical eating disorder, the athlete should be referred to a mental health professional for psychological treatment.[2][64] Depending on the severity, inpatient treatment may be needed. Energy expenditure may also need to be altered by reducing or ceasing exercise.[65] It is believed that normalizing body weight will promote the return of menses and improve bone health.[2][47][66][67][68] When it comes to low BMD, addressing low EA, increasing body weight, having a regular menstrual cycle, and ensuring adequate calcium and vitamin D are recommended.[3]
Role for Physical Therapy in Management[edit | edit source]
A study by Pantano (2009)[69] involved 205 physiotherapists and found that 61% self-reported having knowledge of the Triad and all 3 of its components. However, when actively assessed, only 21% knew the spectrum.[69] Pantano (2009)[69] concluded that physiotherapists need to play a larger role in the prevention of the Triad. Physiotherapists possess the knowledge and skills to help prevent a condition from happening in the first place or increasing in severity. They also help maintain and restore function in those dealing with particular conditions.
Through presentations and discussions, physiotherapists can educate stakeholders in sport on the importance of adopting healthy behaviours and the role physiotherapy plays in preventing and treating the Triad.[69] As mentioned earlier on this page, screening athletes is very important. If there is suspicion of any Triad component, the athlete should be referred onwards to a physician, dietitian, and/or mental health professional for further investigations. This may require some advocacy.
Physiotherapists can play a role in assessing, modifying and monitoring an athlete’s activity, such that they can help place less focus on cardiovascular training.[69] Case studies have shown improvements in bone health after athletes with amenorrhea gained some weight,[70][71] but it is not likely that this will restore BMD by itself.[3] Resistance exercises,[72][73] including weight-training, should also be incorporated 2-3 days a week.[74] While simple low-impact weight-bearing exercise has been shown to increase BMD during menopause, it is likely not enough for younger athletes.[75] Additionally, high-impact sports, including running, may increase an athlete’s risk of developing stress fractures if they do not have adequate BMD to withstand the repeated forces. Lastly, it is also important to note that physiotherapists have the knowledge and skills to recognize and manage stress fractures and osteoporosis. See American College of Sports Medicine Position Stand: Physical Activity and Bone Health.
Since they are part of the multidisciplinary team, physiotherapists will also be working closely with others to determine an athlete’s readiness to return-to-play. If the athlete is not ready to fully return-to-play it is recommended athletes receives a written contract from the physician. The physician will work with each multidisciplinary team members to develop treatment goals and a plan that will allow the athlete to progress. Prior to 2014, there were not any guidelines on clearing an athlete, but something like the Clearance and Return-to-Play Guidelines by Medical Risk Stratification, as well as the Decision-Based Return-to-Play model, that were also created by the De Souza and colleagues (2014)[3] could be incorporated. Click here to access the article and resources for return-to-play.
Differential Diagnosis[edit | edit source]
- Primary Amenorrhea
- Secondary Amenorrhea due to other factors not listed above[57]
- Brittle Bone Disease
- Secondary causes of low BMD (such as B12 Deficiency)
- Thyroid Disorders[58]
- Parathyroid Disorders
- Celiacs Disease[57]
- Osteoporosis - inadequate accumulation of optimal BMD during childhood and adolescence[58]
- Ewing's Sarcoma
More Resources[edit | edit source]
Female Athlete Triad Coalition - http://www.femaleathletetriad.org/
APTA - http://www.moveforwardpt.com/symptomsconditionsdetail.aspx?cid=0ca4bf2e-6d14-4b90-b1ec-ed8ebe13069e
NCAA - http://www.ncaa.org/health-and-safety/sport-science-institute/female-athlete-body-project
References[edit | edit source]
- ↑ Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand: the Female Athlete Triad. Med Sci Sports Exerc. 1997;29(5):i–ix
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 De Souza MJ, Nattiv A, Joy E, Misra M, Williams NI, Mallinson RJ, Gibbs JC, Olmsted M, Goolsby M, Matheson G, Panel E. 2014 Female Athlete Triad Coalition Consensus Statement on treatment and return to play of the female athlete triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014 Feb 1;48(4):289-.
- ↑ 4.0 4.1 4.2 Beals KA, Hill AK. The prevalence of disordered eating, menstrual dysfunction, and low bone mineral density among US collegiate athletes. International journal of sport nutrition and exercise metabolism. 2006 Feb 1;16(1):1-23.
- ↑ 5.0 5.1 Nichols JF, Rauh MJ, Lawson MJ, Ji M, Barkai HS. Prevalence of the female athlete triad syndrome among high school athletes. Archives of pediatrics & adolescent medicine. 2006 Feb 1;160(2):137-42.
- ↑ De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. The Journal of Clinical Endocrinology & Metabolism. 1998 Dec 1;83(12):4220-32.
- ↑ De Souza MJ, Toombs RJ, Scheid JL, O'Donnell E, West SL, Williams NI. High prevalence of subtle and severe menstrual disturbances in exercising women: confirmation using daily hormone measures. Human reproduction. 2010 Feb 1;25(2):491-503.
- ↑ 8.0 8.1 Hoch AZ, Pajewski NM, Moraski L, Carrera GF, Wilson CR, Hoffmann RG, Schimke JE, Gutterman DD. Prevalence of the female athlete triad in high school athletes and sedentary students. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2009 Sep;19(5):421.
- ↑ 9.0 9.1 9.2 9.3 9.4 TORSTVEIT MK, SUNDGOT-BORGEN JO. The female athlete triad exists in both elite athletes and controls. Medicine & Science in Sports & Exercise. 2005 Sep 1;37(9):1449-59.
- ↑ Schneider JE, Wade GN. Inhibition of reproduction in service of energy balance. Reproduction in Context: Social and Environmental Influences on Reproductive Physiology and Behavior. 2000:35-82.
- ↑ De Souza MJ, Williams NI. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Human reproduction update. 2004 Sep;10(5):433-48.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 Gibbs JC, Williams NI, De Souza MJ. Prevalence of individual and combined components of the female athlete triad. Medicine & Science in Sports & Exercise. 2013 May 1;45(5):985-96.
- ↑ Burrows M, Shepherd H, Bird S, Macleod K, Ward B. The components of the female athlete triad do not identify all physically active females at risk. Journal of sports sciences. 2007 Oct 1;25(12):1289-97.
- ↑ Hoch AZ, Stavrakos JE, Schimke JE. Prevalence of female athlete triad characteristics in a club triathlon team. Archives of physical medicine and rehabilitation. 2007 May 1;88(5):681-2.
- ↑ Pollock N, Grogan C, Perry M, Pedlar C, Cooke K, Morrissey D, Dimitriou L. Bone-mineral density and other features of the female athlete triad in elite endurance runners: a longitudinal and cross-sectional observational study. International journal of sport nutrition and exercise metabolism. 2010 Oct 1;20(5):418-26.
- ↑ Schtscherbyna A, Soares EA, de Oliveira FP, Ribeiro BG. Female athlete triad in elite swimmers of the city of Rio de Janeiro, Brazil. Nutrition. 2009 Jun 1;25(6):634-9.
- ↑ Vardar SA, Vardar E, Altun GD, Kurt C, Öztürk L. Prevalence of the female athlete triad in Edirne, Turkey. Journal of sports science & medicine. 2005 Dec;4(4):550.
- ↑ 18.0 18.1 Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. International journal of sport nutrition and exercise metabolism. 2002 Sep 1;12(3):281-93.
- ↑ Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. Journal of science and medicine in sport. 2002 Jun 1;5(2):80-94.
- ↑ Nichols JF, Rauh MJ, Barrack MT, Barkai HS, Pernick Y. Disordered eating and menstrual irregularity in high school athletes in lean-build and nonlean-build sports. International journal of sport nutrition and exercise metabolism. 2007 Aug 1;17(4):364-77.
- ↑ Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. Journal of athletic training. 2005 Jan;40(1):47.
- ↑ Rosendahl J, Bormann B, Aschenbrenner K, Aschenbrenner F, Strauss B. Dieting and disordered eating in German high school athletes and non‐athletes. Scandinavian journal of medicine & science in sports. 2009 Oct;19(5):731-9.
- ↑ 23.0 23.1 Sundgot-Borgen J. Prevalence of eating disorders in elite female athletes. International Journal of Sport Nutrition and Exercise Metabolism. 1993 Mar 1;3(1):29-40.
- ↑ Torstveit MK, Rosenvinge JH, Sundgot‐Borgen J. Prevalence of eating disorders and the predictive power of risk models in female elite athletes: a controlled study. Scandinavian journal of medicine & science in sports. 2008 Feb;18(1):108-18.
- ↑ Torstveit MK, Sundgot-Borgen J. The female athlete triad: are elite athletes at increased risk?. Medicine & Science in Sports & Exercise. 2005 Feb 1;37(2):184-93.
- ↑ Yager J, Devlin MJ, Halmi KA, Herzog DB, Mitchell JE, Powers PS, Zerbe KJ. Guideline watch: Practice guideline for the treatment of patients with eating disorders. Focus. 2005 Oct;3(4):546-51.
- ↑ Johnson C, Powers PS, Dick R. Athletes and eating disorders: the National Collegiate Athletic Association study. International Journal of Eating Disorders. 1999 Sep;26(2):179-88.
- ↑ Sundgot-Borgen J. Nutrient intake of female elite athletes suffering from eating disorders. International Journal of Sport Nutrition and Exercise Metabolism. 1993 Dec 1;3(4):431-42.
- ↑ Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, Maciejewska-Jeske M. Functional hypothalamic amenorrhea and its influence on women’s health. Journal of endocrinological investigation. 2014 Nov 1;37(11):1049-56.
- ↑ 30.0 30.1 Gordon CM. Functional hypothalamic amenorrhea. New England Journal of Medicine. 2010 Jul 22;363(4):365-71.
- ↑ Meczekalski B, Podfigurna-Stopa A, Warenik-Szymankiewicz A, Genazzani AR. Functional hypothalamic amenorrhea: current view on neuroendocrine aberrations. Gynecological Endocrinology. 2008 Jan 1;24(1):4-11.
- ↑ Hind K. Recovery of bone mineral density and fertility in a former amenorrheic athlete. Journal of sports science & medicine. 2008 Sep;7(3):415.
- ↑ Easter A, Treasure J, Micali N. Fertility and prenatal attitudes towards pregnancy in women with eating disorders: results from the Avon Longitudinal Study of Parents and Children. BJOG: An International Journal of Obstetrics & Gynaecology. 2011 Nov;118(12):1491-8.
- ↑ Lambrinoudaki I, Papadimitriou D. Pathophysiology of bone loss in the female athlete. Annals of the New York Academy of Sciences. 2010 Sep;1205(1):45-50.
- ↑ Misra M. What is the best strategy to combat low bone mineral density in functional hypothalamic amenorrhea?. Nature Clinical Practice Endocrinology & Metabolism. 2008 Oct;4(10):542-3.
- ↑ Friday KE, Drinkwater BL, Bruemmer B, CHESNUT 3rd C, Chait A. Elevated plasma low-density lipoprotein and high-density lipoprotein cholesterol levels in amenorrheic athletes: effects of endogenous hormone status and nutrient intake. The Journal of Clinical Endocrinology & Metabolism. 1993 Dec 1;77(6):1605-9.
- ↑ 37.0 37.1 Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Annals of the New York Academy of Sciences. 2006 Dec;1092(1):114-29.
- ↑ Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995 Sep 1;17(3):205-10.
- ↑ Risser WL, Lee EJ, LeBlanc AD, Poindexter HB, Risser JM, Schneider VI. Bone density in eumenorrheic female college athletes. Medicine and science in sports and exercise. 1990 Oct;22(5):570-4.
- ↑ NICHOLS, D. L., and C. F. SANBORN. Female Athlete and Bone. In: Nutrition for Sport and Exercise, J. R. Berning and S. N. Steen. Gaithersburg, Md.: Aspen Publishers, pp. 205–215, 1998.
- ↑ Robinson TL, Snow‐Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. Journal of Bone and Mineral Research. 1995 Jan;10(1):26-35.
- ↑ Juul A, Hagen CP, Aksglaede L, Sørensen K, Mouritsen A, Frederiksen H, Main KM, Mogensen SS, Pedersen AT. Endocrine evaluation of reproductive function in girls during infancy, childhood and adolescence. InPediatric and Adolescent Gynecology 2012 (Vol. 22, pp. 24-39). Karger Publishers.
- ↑ Ihle R, Loucks AB. Dose‐response relationships between energy availability and bone turnover in young exercising women. Journal of bone and mineral research. 2004 Aug;19(8):1231-40.
- ↑ Rumball JS, Lebrun CM. Preparticipation physical examination: selected issues for the female athlete. Clinical Journal of Sport Medicine. 2004 May 1;14(3):153-60.
- ↑ Rumball JS, Lebrun CM. Use of the preparticipation physical examination form to screen for the female athlete triad in Canadian interuniversity sport universities. Clinical Journal of Sport Medicine. 2005 Sep 1;15(5):320-5.
- ↑ Ljungqvist A, Jenoure P, Engebretsen L, Alonso JM, Bahr R, Clough A, De Bondt G, Dvorak J, Maloley R, Matheson G, Meeuwisse W. The International Olympic Committee (IOC) Consensus Statement on periodic health evaluation of elite athletes March 2009. British journal of sports medicine. 2009 Sep 1;43(9):631-43.
- ↑ 47.0 47.1 Committee on Sports Medicine and Fitness. Medical concerns in the female athlete. Pediatrics. 2000 Sep 1;106(3):610-3.
- ↑ Nattiv A, Agostini R, Drinkwater B, Yeager KK. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clinics in sports medicine. 1994 Apr;13(2):405-18.
- ↑ Otis CL. American College of Sports Medicine position stand. The Female Athlete Triad. Med. Sci. Sports Exerc.. 1997;29(5):1669-71.
- ↑ 50.0 50.1 Stickler L, Hoogenboom BJ, Smith L. The Female Athlete Triad‐What Every Physical Therapist Should Know. International journal of sports physical therapy. 2015 Aug;10(4):563.
- ↑ 51.0 51.1 Melin A, Tornberg ÅB, Skouby S, Faber J, Ritz C, Sjödin A, Sundgot-Borgen J. The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br J Sports Med. 2014 Apr 1;48(7):540-5.
- ↑ 52.0 52.1 52.2 52.3 52.4 Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A. The IOC consensus statement: beyond the female athlete triad—Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014 Apr 1;48(7):491-7.
- ↑ 53.0 53.1 53.2 53.3 53.4 53.5 Koltun KJ, Strock NC, Southmayd EA, Oneglia AP, Williams NI, De Souza MJ. Comparison of Female Athlete Triad Coalition and RED-S risk assessment tools. Journal of sports sciences. 2019 Nov 2;37(21):2433-42.
- ↑ 54.0 54.1 54.2 54.3 Holtzman B, Tenforde AS, Parziale AL, Ackerman KE. Characterization of Risk Quantification Differences Using Female Athlete Triad Cumulative Risk Assessment and Relative Energy Deficiency in Sport Clinical Assessment Tool. International journal of sport nutrition and exercise metabolism. 2019 Nov 1;29(6):569-75.
- ↑ 55.0 55.1 55.2 55.3 55.4 55.5 De Souza MJ, Williams NI, Koltun KJ, Strock NC. Female Athlete Triad Coalition risk assessment tool is an evidenced-based tool that is reliable and well-described. Journal of Sports Sciences. 2020 May 2;38(9):996-9.
- ↑ Koltun KJ, Williams NI, De Souza MJ. Female Athlete Triad Coalition Cumulative Risk Assessment Tool: Proposed alternative scoring strategies. Applied Physiology, Nutrition, and Metabolism. 2020 Jun 5(ja).
- ↑ 57.0 57.1 57.2 57.3 57.4 57.5 57.6 57.7 De Souza M, Williams N, Nattiv A, Joy E, Misra M, McComb J, et al. Misunderstanding the female athlete triad: refuting the IOC consensus statement on Relative Energy Deficiency in Sport (RED-S). British Journal Of Sports Medicine [serial on the Internet]. (2014, Oct), [cited March 27, 2017]; 48(20): 1461-1465. Available from: MEDLINE.
- ↑ 58.0 58.1 58.2 58.3 Payne J, Kirchner J. Should you suspect the female athlete triad?. The Journal Of Family Practice [serial on the Internet]. (2014, Apr), [cited March 27, 2017]; 63(4): 187-192. Available from: MEDLINE.
- ↑ The Female Athlete Triad. Medicine & Science In Sports & Exercise [serial on the Internet]. (2007, Oct), [cited March 27, 2017]; 39(10): 1867-1882. Available from: Academic Search Complete.
- ↑ Thein-Nissenbaum J. Long term consequences of the female athlete triad. Maturitas [serial on the Internet]. (2013, June), [cited March 27, 2017]; 75(2): 107-112. Available from: MEDLINE.
- ↑ Zach KN, Machin AL, Hoch AZ. Advances in management of the female athlete triad and eating disorders. Clinics in sports medicine. 2011 Jul 1;30(3):551-73.
- ↑ Nazem TG, Ackerman KE. The female athlete triad. Sports Health. 2012 Jul;4(4):302-11.
- ↑ Papanek PE. The female athlete triad: An emerging role for physical therapy. J Orthop Sports Phys Ther.
- ↑ Temme KE, Hoch AZ. Recognition and rehabilitation of the female athlete triad/tetrad: a multidisciplinary approach. Current Sports Medicine Reports. 2013 May 1;12(3):190-9.
- ↑ Mountjoy M, Sundgot-Borgen JK, Burke LM, Ackerman KE, Blauwet C, Constantini N, Lebrun C, Lundy B, Melin AK, Meyer NL, Sherman RT. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. British Journal of Sports Medicine. 2018 May 15.
- ↑ Arends JC, Cheung MY, Barrack MT, Nattiv A. Restoration of menses with nonpharmacologic therapy in college athletes with menstrual disturbances: a 5-year retrospective study. International journal of sport nutrition and exercise metabolism. 2012 Apr 1;22(2):98-108.
- ↑ Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. The Journal of Clinical Endocrinology & Metabolism. 2008 Apr 1;93(4):1231-7.
- ↑ Audí L, Vargas DM, Gussinyé M, Yeste D, Martí G, Carrascosa A. Clinical and biochemical determinants of bone metabolism and bone mass in adolescent female patients with anorexia nervosa. Pediatric research. 2002 Apr;51(4):497-504.
- ↑ 69.0 69.1 69.2 69.3 69.4 Pantano KJ. Strategies used by physical therapists in the US for treatment and prevention of the female athlete triad. Physical Therapy in Sport. 2009 Feb 1;10(1):3-11.
- ↑ ZANKER CL, COOKE CB, TRUSCOTT JG, OLDROYD B, JACOBS HS. Annual changes of bone density over 12 years in an amenorrheic athlete. Medicine & Science in Sports & Exercise. 2004 Jan 1;36(1):137-42.
- ↑ Fredericson M, Kent K. Normalization of bone density in a previously amenorrheic runner with osteoporosis. Medicine & Science in Sports & Exercise. 2005 Sep 1;37(9):1481-6.
- ↑ Martyn-St James M, Carroll S. Progressive high-intensity resistance training and bone mineral density changes among premenopausal women. Sports Medicine. 2006 Aug 1;36(8):683-704.
- ↑ Wallace BA, Cumming RG. Systematic review of randomized trials of the effect of exercise on bone mass in pre-and postmenopausal women. Calcified Tissue International. 2000 Jul 1;67(1):10-8.
- ↑ Martyn-St James M, Carroll S. Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. Journal of bone and mineral metabolism. 2010 May 1;28(3):251-67.
- ↑ Papanek PE. The female athlete triad: an emerging role for physical therapy. Journal of Orthopaedic & Sports Physical Therapy. 2003 Oct;33(10):594-614.