Autonomic Nervous System and Spinal Cord Injury
Original Editor - Melanie Harding
Top Contributors - Ewa Jaraczewska, Jess Bell and Kim Jackson
Introduction[edit | edit source]
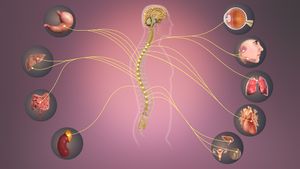
The autonomic nervous system is a component of the peripheral nervous system that regulates involuntary physiological processes, including heart rate, blood pressure, respiration, digestion, and sexual arousal. It contains three anatomically distinct divisions: the sympathetic, parasympathetic, and enteric nervous systems. Impairments of the autonomic nervous system in spinal cord injury may result in autonomic dysfunction.[1] This article provides a comprehensive review of the impact of spinal cord injury on the autonomic nervous system.
Anatomy of the Autonomic Nervous System (ANS)[edit | edit source]
There are two main functions of the autonomic nervous system:
- regulating visceral functions
- maintaining homeostasis within the human body
Sympathetic Nervous System (SNS)[edit | edit source]
The sympathetic nervous system (SNS) arises from the thoracolumbar regions of the spinal cord. There are three components in the sympathetic pathway: preganglionic neurons, sympathetic ganglia and postganglionic neurons.[2] The preganglionic cell bodies are located in the intermediolateral horns of the spinal cord. The sympathetic nerves run parallel to the spinal cord on both sides of the vertebral column.[2]
The functions of the SNS include the following:
- "fight or flight" response, which includes increased blood pressure and heart rate, increased glucose production, and cessation of gastrointestinal peristalsis[3]
- fibres from the SNS innervate many organs: the SNS provides physiological regulation over diverse body processes, including pupil diameter, gut motility (movement), and urinary output[4]
- preparing the body for physical activity: there is "a whole-body reaction affecting many organ systems throughout the body to redirect oxygen-rich blood to areas of the body needed during intense physical demand"[4]
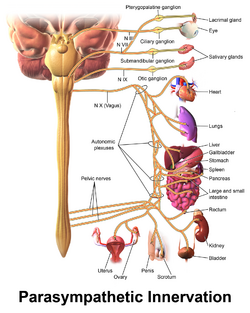
Parasympathetic Nervous System (PNS)[edit | edit source]
The preganglionic cell bodies of the parasympathetic nervous system (PNS) originate at the craniosacral axis. The PNS has long efferent preganglionic fibres but short postganglionic fibres to the effector organs.[5] The PNS innervates the head, viscera, and external genitalia.
Functions of the PNS include:[3]
- promoting "rest and digest"
- lowering heart rate and blood pressure
- facilitating gastrointestinal peristalsis
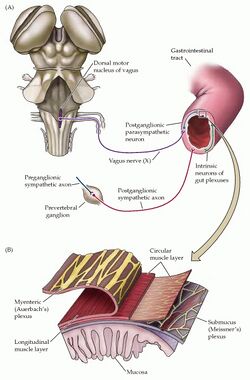
Enteric Nervous System (ENS)[edit | edit source]
The enteric nervous system (ENS) is the largest, most complex unit within the peripheral nervous system. It innervates the gastrointestinal tract through a network of ganglion-rich nerve connections, extending from the oesophagus to the anal canal.[6][7] While the ENS receives input from the central nervous system (CNS), it can act independently from the CNS because of local reflex circuits.
Functions of the ENS include:[6]
- propulsion of food
- blood flow regulation
- nutrient handling
- immunological defence
Autonomic Nervous System and Spinal Cord Injury (SCI)[edit | edit source]
Spinal cord injury can affect all three subsystems of the autonomic nervous system due to their anatomical location, loss of supraspinal influence, and sustained responses to afferent stimuli.[5] A number of pathophysiological responses from the ANS can occur after SCI, contributing to various complications and comorbidities.[5][3]
- Cardiovascular dysfunction: SCI (particularly cervical and high thoracic injuries) can result in parasympathetic dominance and reduced sympathetic influence (i.e. sympathetic blunting). This imbalance can lead to various cardiovascular complications, including:
- low resting arterial blood pressure
- postural hypotension
- autonomic dysreflexia (sudden, acute hypertension in response to specific stimuli)
- bradycardia or arrhythmia
- Thermoregulatory dysfunction: commonly occurs in individuals with high thoracic and cervical SCI, primarily because of sympathetic dysfunction or blunting. This affects the ability of the body to redistribute warm blood from the core to the periphery and inhibits sweating below the level of injury. When the body attempts to dissipate heat, excess sweating may occur, and the skin may appear flushed above the injury.[5] Thermoregulatory dysfunction is associated with:[5]
- poikilothermia[5]
- an individual with an SCI takes on "the ambient temperature around them due to the inability to regulate core body temperature"
- quad fever (idiopathic hyperpyrexia)
- "an extreme elevation in body core temperature beyond 40.8°C (105.4°F) in a patient with spinal cord injury"[8]
- exercise-induced fever
- hyperhidrosis (excess sweating) or hypohidrosis (reduced sweating)
- poikilothermia[5]
- Respiratory dysfunction: associated with parasympathetic dominance and blunted supraspinal sympathetic drive in individuals with high cervical and thoracic SCI:[5]
- bronchiolar constriction: associated with unopposed vagal influence on the bronchial tree, as well as hyper-reactive airways and increased secretion of mucus
- individuals with high thoracic and cervical SCI are unable to cough / clear pulmonary secretions voluntarily and are at risk of:
- atelectasis
- pneumonia
- mucus plugging
- during exercise, inspiratory effort is limited by ventilatory insufficiency, bronchoconstriction and mucus production
- Gastrointestinal dysfunction: while the ENS can act independently, it is usually influenced by the SNS and PNS. PNS dominance and sympathetic blunting in SCI can lead to:[5]
- acute and chronic increases in gastric acid secretions
- high rates of biliary sludge, cholelithiasis and cholecystitis
- increased transit time at the distal colon
- reflex colorectal contractions
- constipation or bowel incontinence
- Genitourinary dysfunction: after SCI, there is increased uninhibited activation of the sympathetic and parasympathetic systems and the somatic nervous system, responsible for bladder storage and emptying. The results of genitourinary dysfunction include:[5]
- bladder and bowel dysfunction
- detrusor sphincter dyssynergia: high bladder pressures and likely vesicoureteral reflux, which is associated with hydroureter, hydronephrosis and urinary incontinence
- impaired sexual function affecting fertility (usually preserved in females), arousal, lubrication, ejaculation, and orgasm
- problems during pregnancy, labour, and breastfeeding
- bladder and bowel dysfunction
For more information on these pathophysiological responses, please see Autonomic Dysfunction and Management after Spinal Cord Injury: A Narrative Review.
Pathophysiological responses from the ANS can occur during procedures or when pain or injury occurs, such as during:[3]
- surgery
- invasive investigational procedures such as urodynamic studies
- stretches
- fractures
- urinary tract infection (UTI)
Assessment of the Autonomic Nervous System in Spinal Cord Injury[edit | edit source]
The International Standards to document Autonomic Function following SCI (ISAFSCI) was published in 2009 and revised in 2012. The ISAFSCI is also referred to as the Autonomic Standards. The American Spinal Injury Association (ASIA) and the International Spinal Cord Society (ISCoS) recommendations for the Autonomic Standards include the following:[9]
- using Autonomic Standards in conjunction with the full International Standards for Neurological Classification of SCI (ISNCSCI) following the initial injury
- tracking the association between changes in ANS function "correspondent with changes in the neurological level of injury (NLI) and completeness of injury as classified by the ASIA Injury Severity (AIS) scale"[9]
- tracking changes in autonomic functions following clinical interventions or during clinical trials
The Autonomic Standards Assessment Form includes the following:[3]
- autonomic control of the heart
- autonomic control of blood pressure
- autonomic control of sweating
- temperature regulation
- autonomic and somatic control of a bronchopulmonary system
- lower urinary tract
- bowel and sexual function
- urodynamics
You can learn more about the impact of ANS impairment on the function of organ systems and how to use the ISAFSCI at the ASIA e-Learning Center.
Function of the Autonomic Nervous System in Spinal Cord Injury[edit | edit source]
Autonomic Function Assessment[edit | edit source]
The cardiovascular, thermoregulation, sudomotor control, bronchopulmonary and lower urinary tract systems are assessed, and impairments are documented.[3]
| Scoring:
2 = Normal Function 1 = Reduced/AlteredFunction 0 = Complete loss of control NT = not tested |
||||
|---|---|---|---|---|
| Normal (2) | Reduced/Altered (1) | Complete loss of control (0) | Comments | |
| Heart rate | 61-99 beats per minute (bpm) |
|
| |
| Systolic blood pressure (SBP) | 91-138mmHg |
|
| |
| Diastolic blood pressure (DBP) | 62-89 mmHg |
|
| |
| Core body temperature | 36.4-37.6°C
(97.5-99.7 °F) |
|
Temperature measurements must be taken under the following conditions:
| |
| Sudomotor control | Sweating over all the skin surfaces |
|
|
|
| Respiration | Normal voluntary breathing |
|
|
|
| Lower urinary tract |
|
| ||
Temperature Regulation[edit | edit source]
Individuals with a lesion at T6 and above:
- lack the descending sympathetic control to respond appropriately to environmental changes in temperature[3]
- become adversely affected by the lack of afferent and efferent thermoregulatory information[10]
- are more affected by internal and external heat load, resulting in a greater potential for exercise-induced hyperthermia[10]
- sweating, vasodilation, and faster breathing are less effective measures to reduce heat as there is no response below the lesion; this can lead to fever[3]
The following techniques can be successful in restoring normal body temperature in individuals with SCI:[10]
- wearing cooling garments or other cooling devices
- cooling vest
- refrigerated headpiece
- cooling device on the feet
- water spray and artificial sweat
- ice slurry
- using a cold cloth or a fan[3]
Orthostatic Hypotension[edit | edit source]
Orthostatic hypotension occurs due to the interruption of efferent pathways from the brain stem's vasomotor centre to the sympathetic nerves involved in vasoconstriction:[3]
- symptoms may include dizziness, nausea, light-headedness, or faintness
- physical signs include paleness, excessive sweating, or a loss of consciousness
Autonomic Nervous System Symptom Management in Spinal Cord Injury[edit | edit source]
General Rules[edit | edit source]
Be aware...........
- of the system impairments in SCI - educate yourself and your patients
- of the less obvious or even silent symptoms
- that suctioning or insertion of a nasogastric tube can commonly induce bradycardia and apnoea in individuals with SCI
Autonomic Dysreflexia[edit | edit source]
"Sudden bouts of hypertension triggered by noxious afferent stimuli below the level of lesion, above the outflow to the splanchnic and renal vascular beds (T5– T6)"[3] -- Melanie Harding
Noxious afferent stimuli below the level of the lesion can cause the following:[3]
- widespread activation of the sympathetic nervous system, demonstrated by the increased release of norepinephrine (noradrenaline)
- vasoconstriction in the majority of vascular beds below the level of injury: muscle, skin, kidneys, and presumably also the splanchnic vascular bed
- an increase in arterial blood pressure, which activates the baroreceptors; this acts to buffer the vasoconstriction through dilation of vascular beds above the lesion level (where there is intact central control) and through a reduction in heart rate (vagal innervation to the heart is unaffected by SCI)
Signs of Autonomic Dysreflexia[edit | edit source]
The signs of AD include:
- piloerection (goosebumps)
- chills or shivering
- pounding headache
- paraesthesia
- flushed skin
- diaphoresis (sweating) above the lesion level
- nasal congestion
- anxiety (feeling of impending doom)
- malaise
- nausea
Please watch this optional video if you would like to learn more about Autonomic Dysreflexia:
Triggering Factors for Autonomic Dysreflexia[edit | edit source]
The following factors can trigger autonomic dysreflexia in individuals with SCI:
- irritation of the urinary bladder
- catheterisation and manipulation or blockage of an indwelling catheter
- urinary tract infection
- detrusor sphincter dyssynergia and bladder percussion
- distended / overfull bladder
- gastrointestinal tract distension, constipation, anal fissures, haemorrhoids.
- stimuli that would be noxious if pain sensation was preserved, such as:
- bone fractures or joint displacements, pressure sores, ingrown toenails
- sexual activity / orgasm may induce autonomic dysreflexia in both sexes
- during pregnancy, delivery and breastfeeding
- cystoscopy, urodynamic studies (UDS), vibration or electrostimulation for ejaculation
- electrical stimulation or stretching of muscles or neural tissue
Management of Autonomic Dysreflexia[edit | edit source]
Management of autonomic dysreflexia includes the following:
- sit the patient up
- loosen any tight clothing or constrictive devices
- rule out a bladder problem
- if no urinary catheter is in place, catheterise the patient
- if there is a urinary catheter, check the system along its entire length for blockages, twists, kinks or obstructions and correct placement
- if the catheter seems to be blocked, report this to the nurse or carer, so they can gently irrigate the bladder with a small amount of body temperature normal saline
- rule out faecal impaction
- the carer / nurse should check the rectum for stool, using a local anaesthetic jelly as a lubricant
- gentle manual evacuation is recommended
- if no obvious cause is found, emergency medical care is advised
- monitor blood pressure and pulse every 2-5 minutes until the patient has stabilised
- an antihypertensive agent is recommended when the systolic blood pressure is at or above 150 mm Hg; medicating with a short-acting antihypertensive is of the utmost importance
- the individual's symptoms and blood pressure should be monitored for at least 2 hours after the episode to ensure there is no further elevation in blood pressure
Complications of Unmanaged Autonomic Dysreflexia[edit | edit source]
When untreated, autonomic dysreflexia can cause complications / comorbidities or mortality in patients with a spinal cord injury, including:
- pulmonary embolism
- stroke
- combined immunodeficiency (CIDS)
- clinically significant infectious complications
- seizures
- retinal haemorrhage or detachment
- pulmonary oedema
- renal insufficiency
- myocardial infarction
Resources[edit | edit source]
- Fleming MA 2nd, Ehsan L, Moore SR, Levin DE. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol Res Pract. 2020 Sep 8;2020:8024171.
- Henke AM, Billington ZJ, Gater DR Jr. Autonomic Dysfunction and Management after Spinal Cord Injury: A Narrative Review. J Pers Med. 2022 Jul 7;12(7):1110.
- ASIA e-Learning Center
- General Autonomic Function
References[edit | edit source]
- ↑ Wulf MJ, Tom VJ. Consequences of spinal cord injury on the sympathetic nervous system. Front Cell Neurosci. 2023 Feb 28;17:999253.
- ↑ 2.0 2.1 Ken Hub. Sympathetic NS. Available from:https://www.kenhub.com/en/library/anatomy/sympathetic-nervous-system (accessed 17.3.2024)
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Harding M. Autonomic Nervous System and Spinal Cord Injury Course. Plus, 2024.
- ↑ 4.0 4.1 Alshak MN, M Das J. Neuroanatomy, Sympathetic Nervous System. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542195/ [last access 17.03.2024]
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Henke AM, Billington ZJ, Gater DR Jr. Autonomic Dysfunction and Management after Spinal Cord Injury: A Narrative Review. J Pers Med. 2022 Jul 7;12(7):1110.
- ↑ 6.0 6.1 Fleming MA 2nd, Ehsan L, Moore SR, Levin DE. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol Res Pract. 2020 Sep 8;2020:8024171.
- ↑ Wang H, Foong JPP, Harris NL, Bornstein JC. Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal Immunol. 2022 Jan;15(1):27-39.
- ↑ Ali S, Ganesan D, Sundaramoorthy V. Quad fever in a case of cervical cord injury-a rare case report. Asian J Neurosurg. 2022 Jun 28;17(1):85-87.
- ↑ 9.0 9.1 Wecht JM, Krassioukov AV, Alexander M, Handrakis JP, McKenna SL, Kennelly M, Trbovich M, Biering-Sorensen F, Burns S, Elliott SL, Graves D, Hamer J, Krogh K, Linsenmeyer TA, Liu N, Hagen EM, Phillips AA, Previnaire JG, Rodriguez GM, Slocum C, Wilson JR. International Standards to document Autonomic Function following SCI (ISAFSCI): Second Edition. Top Spinal Cord Inj Rehabil. 2021 Spring;27(2):23-49
- ↑ 10.0 10.1 10.2 Grossmann F, Flueck JL, Perret C, Meeusen R, Roelands B. The Thermoregulatory and Thermal Responses of Individuals With a Spinal Cord Injury During Exercise, Acclimation and by Using Cooling Strategies-A Systematic Review. Front Physiol. 2021 Apr 1;12:636997.
- ↑ Dr Matt & Dr Mike. Autonomic Dysreflexia. Available from:https://www.youtube.com/watch?v=eocOmytfg8s [last accessed 18/3/2024]