Remote Screening for Lumbar Spine Red Flags: Difference between revisions
No edit summary |
Bruno Serra (talk | contribs) m (Removed link to a pdf resource.) |
||
| (47 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
'''Introduction''' | <div class="editorbox"> '''Original Editor '''- [[User:Tim Kirby|Tim Kirby]], [[User:Nathan May|Nathan May]],[[User:Zak Jackson|Zak Jackson]] and [[User:Adam Auckland]] as part of the [[Nottingham University Spinal Rehabilitation Project]]<br> | ||
'''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}</div> | |||
== Introduction == | |||
[[Red Flags in Spinal Conditions|Red flags]] are clinical findings that are identified from a patient's medical history and the clinical exam and can increase the suspicion of serious pathology such as an infection, cancer, or a fracture<ref name=":7">Greenhalgh S, Finucane LM, Mercer C, Selfe J. [https://www.sciencedirect.com/science/article/abs/pii/S2468781220303131 Safety netting; best practice in the face of uncertainty. Musculoskeletal Science and Practice.] 2020 May 12:102179.</ref>. The presence of red flags from a patient's subjective and objective assessment are thought to put them at a higher risk of '''serious pathology''' and warrant referral for further diagnostic testing.<ref>Delitto, A., George, S. and Godges. J, 2012. [https://www.jospt.org/doi/10.2519/jospt.2012.42.4.A1 Low Back Pain Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health from the Orthopaedic Section of the American Physical Therapy Association]. Journal of orthopaedics and sports physical therapy. 42(4), pp. 57</ref> | |||
It is vital that practitioners are aware of these red flags as they form a key component of the assessment and management of [[Low Back Pain|low back pain]] whilst increasing patient safety.<ref name=":0">Ferguson FC, Morison S, Ryan CG. [https://onlinelibrary.wiley.com/doi/abs/10.1002/msc.1079 Physiotherapists' understanding of red flags for back pain.] Musculoskeletal care. 2015 Mar;13(1):42-50.</ref> | |||
== Red Flags and Low Back Pain == | |||
The role of physiotherapists as primary identifiers of red flags has grown owing to the spread of self‐referral services.<ref>Holdsworth LK, Webster VS, McFadyen AK, Scottish Physiotherapy Self Referral Study Group. [https://www.csp.org.uk/journal/article/physiotherapy-march-2006/self-referral-physiotherapy-deprivation-geographical Self-referral to physiotherapy: deprivation and geographical setting: is there a relationship? Results of a national trial.] Physiotherapy. 2006 Mar 1;92(1):16-25.</ref> Physiotherapists often exist without any medical input or review.<ref>Kersten P, McPherson K, Lattimer V, George S, Breton A, Ellis B. [https://www.csp.org.uk/journal/article/physiotherapy-december-2007/physiotherapy-extended-scope-practice-who-doing-what Physiotherapy extended scope of practice–who is doing what and why?.] Physiotherapy. 2007 Dec 1;93(4):235-42.</ref> <ref>McPherson K, Kersten P, George S, Lattimer V, Breton A, Ellis B, Kaur D, Frampton G. [https://pubmed.ncbi.nlm.nih.gov/17018199/ A systematic review of evidence about extended roles for allied health professionals.] Journal of health services research & policy. 2006 Oct 1;11(4):240-7.</ref>Therefore, there is a need to ensure that physiotherapists have a good understanding of individual red flags, understand their importance, and can ask these questions in a clear and unambiguous manner. Similarly, physiotherapists must have a clear understanding and agreed pathways of care dependent on these findings. Failure to do so raises issues around patient safety and professional reputation.<ref name=":0" /> | |||
One study by Feguson et al. (2015) aimed to investigate the red flags that are routinely recorded by physiotherapists<ref name=":0" />. This included which red flags do they consider to be most important, how would they define each red flag, and how they would ask each red‐flag question to a person with back pain. 98 physiotherapists responded to the survey, 84% worked exclusively in the National Health Service (NHS). They recorded that ‘Previous history of '''cancer, ‘saddle anesthesia, and difficulty with micturition were the red flags that raised suspicion of serious pathology the most'''. The physiotherapists involved in the study stated the following way to ask about red flags: | |||
* '''History of cancer''': an individual who has previously been diagnosed with cancer. | |||
* '''Saddle anesthesia:''' Since your symptoms commenced, have you noticed any pins and needles or numbness around your back passage or genital area. | |||
Finally, the limited consensus was found in how physiotherapists asked patients about red flags. However, one theme, in particular, emerged, which is the use of nebulous terminology - for example, the terms recent, weight loss, and prolonged period.<ref name=":0" /> | |||
== COVID-19 and Remote Consultations == | |||
[[Coronavirus Disease (COVID-19)|COVID-19]] is an infectious respiratory disease, caused by the SARS-Cov-2 virus ([[SARS Severe Acute Respiratory Syndrome|Severe Acute Respiratory Syndrome]] Coronavirus Two) which spreads primarily through saliva and respiratory droplets when an infected person sneezes or coughs. Although the majority of people infected will only experience mild to moderate respiratory illness, the elderly and those with underlying health conditions are more likely to suffer from severe illness. As at November 2020 there have been over 61 million confirmed cases that have resulted in over 1.4 million deaths globally<ref>World Health Organisation: [https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Coronavirus Disease (COVID-19) Pandemic]. [Last accessed 29 November 2020]</ref>. | |||
The reproduction number (R-value) is a method used to identify the disease's ability to spread meaning, this value represents the number of people that one infected person will pass the virus on to, on average.<ref>Yi Y, Lagniton PN, Ye S, Li E, Xu RH. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7098028/ COVID-19: what has been learned and to be learned about the novel coronavirus disease.] International journal of biological sciences. 2020;16(10):1753.</ref> R-value >1.0 means the disease will spread exponentially whereas, an R-value <1.0 means the disease will spread slowly and eventually die out.<ref>Shim E, Tariq A, Choi W, Lee Y, Chowell G. [https://www.sciencedirect.com/science/article/pii/S1201971220301508 Transmission potential and severity of COVID-19 in South Korea.] International Journal of Infectious Diseases. 2020 Mar 18.</ref> The R-value is estimated to be between 1.4 and 2.5 which makes this disease significantly more contagious than the influenza virus.<ref>Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. T[https://academic.oup.com/jtm/article/27/2/taaa021/5735319 he reproductive number of COVID-19 is higher compared to SARS coronavirus.] Journal of travel medicine. 2020 Mar 13.</ref> This has forced healthcare services to rapidly implement alternative models of care to avoid face-to-face contact between patient and clinician.<ref>Greenhalgh T, Wherton J, Shaw S, Morrison C. [https://www.bmj.com/content/368/bmj.m998 Video consultations for covid-19.]</ref> Therefore, guidelines have been released for remote consultations based on current evidence evaluating its effectiveness.<ref>NHS.uk. 2020. GP Online And Video Consultations. [online] Available at: [https://www.nhs.uk/using-the-nhs/nhs-services/gps/gp-online-and-video-consultations/ <https://www.nhs.uk/using-the-nhs/nhs-services/gps/gp-online-and-video-consultations/]> [Accessed 20 April 2020].</ref> | |||
A systematic review (SR) compared the effectiveness of '[[Telerehabilitation and Smartphone Apps in Physiotherapy|telerehabilitation']] (defined by phone call and/or video consultations) and face-to-face consultations within a physiotherapy musculoskeletal (MSK) setting and result support its effectiveness.<ref>Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. [https://journals.sagepub.com/doi/abs/10.1177/0269215516645148 Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis.] Clinical rehabilitation. 2017 May;31(5):625-38.</ref><ref>Shukla H, Nair SR, Thakker D. [https://pubmed.ncbi.nlm.nih.gov/26843466/ Role of telerehabilitation in patients following total knee arthroplasty: Evidence from a systematic literature review and meta-analysis.] Journal of telemedicine and telecare. 2017 Feb;23(2):339-46.</ref> | |||
Qualitative studies have also provided insight into patient and clinician experiences during remote consultations. Positive feedback included time convenience, cost efficiency, easy to use technology, home environments, empowering self-management, and taking away the stigmatization of ‘hands-on’ therapy for treating low back pain.<ref>Hinman RS, Nelligan RK, Bennell KL, Delany C. “[https://europepmc.org/article/med/28217864 Sounds a bit crazy, but it was almost more personal:” a qualitative study of patient and clinician experiences of physical therapist–prescribed exercise for knee osteoarthritis via Skype.] Arthritis care & research. 2017 Dec;69(12):1834-44.</ref><ref>Synnott A, O’Keeffe M, Bunzli S, Dankaerts W, O'Sullivan P, O'Sullivan K. [https://pubmed.ncbi.nlm.nih.gov/25812929/ Physiotherapists may stigmatise or feel unprepared to treat people with low back pain and psychosocial factors that influence recovery: a systematic review.] Journal of physiotherapy. 2015 Apr 1;61(2):68-76.</ref> | |||
This evidence supports the use of remote consultations. Therefore, it is important to discuss how MSK physiotherapists can successfully identify and screen patients for red flags when presenting with low back pain during remote consultations, given the current global pandemic. | |||
== Low Back Pain Red Flags == | |||
Back pain is very common in the UK. It has a lifetime chance of occurrence of 59%.<ref>GP online. 2008. Red flag symptoms: Back pain [Online]. Available from: https://www.gponline.com/red-flag-symptoms-back-pain/musculoskeletal-disorders/musculoskeletal-disorders/article/798743 [Accessed 20/05/20]</ref> The majority of back pain clears up quite quickly; however, back pain experienced along with ‘red flag’ symptoms may have a serious underlying cause. | |||
This page discusses the clinical indicators for: | |||
# [[Cauda Equina Syndrome]] | |||
# [[Spinal Malignancy|Malignancy]] | |||
# [[Lumbar Spine Fracture|Vertebral Fractures]] | |||
# [[Communicable Diseases|Infection]] | |||
=== Cauda Equina === | |||
[[Cauda Equina Syndrome|Cauda equina syndrome (CES)]] is a rare but potentially devastating neurological condition affecting the nerve roots at the lower end of the [[Spinal cord anatomy|spinal cord]] known as the cauda equina (CE). The CE is responsible for the innervation of the lower limbs, control of the anal sphincter, regulation, and function of the bladder and distal bowel and sensation to the skin around the bottom and back passage. | |||
' | 'A patient presenting with acute back pain and/or leg pain with a suggestion of a disturbance of their bladder or bowel function and/or saddle sensory disturbance should be suspected of having a CES. Most of these patients will not have critical compression of the cauda equina. However, in the absence of reliably predictive symptoms and signs, there should be a low threshold for investigation with an emergency scan’.<ref>Germon T, Ahuja S, Casey ATH, Todd NV, Rai A. [https://pubmed.ncbi.nlm.nih.gov/25708139/ British Association of Spine Surgeons standards of care for cauda equina syndrome.] Spine J. 2015 Mar 2;15(3 Suppl):S2-S4. doi: 10.1016/j.spinee.2015.01.006. PMID: 25708139.</ref> | ||
There are many causes of CES | ===== Epidemiology ===== | ||
There are many causes of CES with the most common being that of a lumbar spine [[Disc Herniation|disc herniation.]] It occurs most frequently between the ages of 31–50.<ref>Fuso FA, Dias AL, Letaif OB, Cristante AF, Marcon RM, Barros Filho TE. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3861993/ Epidemiological study of cauda equina syndrome.] Acta ortopedica brasileira. 2013 Jun;21(3):159-62.</ref> Cauda equina compression usually occurs at the level L4/5.<ref>Fraser S, Roberts L, Murphy E. [https://pubmed.ncbi.nlm.nih.gov/19887225/ Cauda equina syndrome: a literature review of its definition and clinical presentation.] Arch Phys Med Rehabil. 2009 Nov;90(11):1964-8. doi: 10.1016/j.apmr.2009.03.021. PMID: 19887225.</ref> Although disc herniation is the most common mechanism, CES can be caused by any space-occupying lesion, such as [[Lumbar Spinal Stenosis|spinal stenosis]], tumor, cysts, infection, or bony ingress can narrow the spinal canal and cause compression of the cauda equina.<ref>Greenhalgh S, Finucane L, Mercer C, Selfe J. [https://pubmed.ncbi.nlm.nih.gov/29935940/ Assessment and management of cauda equina syndrome.] Musculoskelet Sci Pract. 2018 Oct;37:69-74. doi: 10.1016/j.msksp.2018.06.002. Epub 2018 Jun 7. PMID: 29935940.</ref> | |||
Published estimates of the incidence for CES are fewer than one per 100 000 population | Published estimates of the incidence for CES are fewer than one per 100 000 population.<ref>Podnar S. [https://onlinelibrary.wiley.com/doi/abs/10.1002/mus.20696 Epidemiology of cauda equina and conus medullaris lesions]. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2007 Apr;35(4):529-31.</ref> | ||
In a primary care setting, | In a primary care setting, Table 1 highlights the incidence of diagnosed patients with CES in the UK 2018/19.<ref name=":2">Barnes, M., 2019. NHS Digital, Hospital Episode Statistics For England. Outpatient Statistics, 2018 - 2019.. Primary Diagnosis by Attendance Type. NHS Digital.</ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
|'''Condition''' | |'''Condition''' | ||
| Line 42: | Line 61: | ||
|58 | |58 | ||
|0.0% | |0.0% | ||
|} | |} | ||
===== Prognosis ===== | |||
The prognosis for complete recovery is dependent upon many factors. The most important of these is the severity and duration of compression upon the damaged nerve(s). Generally, the longer the time before the intervention to remove the compression causing nerve damage, the greater the damage caused to the nerve(s). Similarly, Kennedy et al. (1999) describe the most important factor identified in a series of predictors for a favorable outcome in CES was an early diagnosis.<ref>Kennedy JG, Soffe KE, McGrath A, Stephens MM, Walsh MG, McManus F. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3611188/ Predictors of outcome in cauda equina syndrome.] European Spine Journal. 1999 Aug 1;8(4):317-22.</ref> This highlights the importance of understanding and identifying red flags. | |||
The | ===== Clinical Indicators ===== | ||
The subjective history is the most important aspect of the examination, particularly early in the presentation of a patient with CES as the imperceptible and possible vague symptoms related to early CES need to be identified using clear and unmistakable methods of communication.<ref>Bin MA, Hong WU, Jia LS, Wen YU, Shi GD, Shi JG. [https://pubmed.ncbi.nlm.nih.gov/19493474/ Cauda equina syndrome: a review of clinical progress.] Chinese medical journal. 2009 May 1;122(10):1214-22.</ref> <ref>Sun JC, Xu T, Chen KF, Qian W, Liu K, Shi JG, Yuan W, Jia LS. [https://europepmc.org/article/med/24150427 Assessment of cauda equina syndrome progression pattern to improve diagnosis.] Spine. 2014 Apr 1;39(7):596-602.</ref> | |||
Premkumar et al. (2018) reported that the combination of recent loss of bladder control and recent loss of bowel control produced a specificity of 97.4%<ref name=":3" />. Both studies highlighted that while the specificity was generally high, all red flag questions had poor sensitivity when identifying their diagnoses of interest. | |||
Premkumar et al. (2018) | |||
{| class="wikitable" | {| class="wikitable" | ||
|'''Indicator''' | |'''Indicator''' | ||
| Line 73: | Line 89: | ||
|} | |} | ||
Table 2 - Clinical Indicators from Premkumar et al. (2018)<ref name=":3">Premkumar A, Godfrey W, Gottschalk MB, Boden SD. [https://europepmc.org/article/med/29509613 Red flags for low Back pain are not always really red: a prospective evaluation of the clinical utility of commonly used screening questions for low Back pain.] JBJS. 2018 Mar 7;100(5):368-74.</ref>. | |||
{| class="wikitable" | {| class="wikitable" | ||
|'''Indicator''' | |'''Indicator''' | ||
| Line 96: | Line 113: | ||
|} | |} | ||
' | Table 3 - Clinical Indicators from Tsiang et al. (2019).<ref name=":4">Tsiang JT, Kinzy TG, Thompson N, Tanenbaum JE, Thakore NL, Khalaf T, Katzan IL. [https://pubmed.ncbi.nlm.nih.gov/29959102/ Sensitivity and specificity of patient-entered red flags for lower back pain.] The Spine Journal. 2019 Feb 1;19(2):293-300.</ref> | ||
During screening for red flags, Greenhalgh et al. (2015) in their qualitative investigation of patient's experience of CES found that one of the key problems in communication was the technical/medical language used by clinicians. For example, ‘saddle numbness’ to a patient is not clearly understood. The patient participants in the study emphasized the need for clinicians to use clear and some would say ‘explicit language’ that can be readily understood during a consultation.<ref>Greenhalgh S, Truman C, Webster V, Selfe J. [https://content.iospress.com/articles/physiotherapy-practice-and-research/ppr047 An investigation into the patient experience of Cauda Equina Syndrome: A qualitative study.] Physiotherapy Practice and Research. 2015 Jan 1;36(1):23-31.</ref> | |||
{| class="wikitable" | |||
|+ | |||
!Green | |||
(Low Risk) | |||
!Amber | |||
(Intermediate Risk) | |||
!Red | |||
(High Risk) | |||
|- | |||
|Increased level of suspicion – Continue with assessment | |||
|Further increase in level of suspicion – Clinically Reason Between Option 1 and Option 2. | |||
Option 1: Physiotherapy Appointment and/or GP referral Necessary | |||
Option 2: Urgent Physiotherapy Appointment and/or Urgent GP referral Necessary | |||
|Further increase in level of suspicion for Cauda Equina Syndrome – Do Not continue with assessment Urgent A&E Referral Necessary | |||
|- | |||
|Neurological: | |||
* Weakness in limbs | |||
|Neurological | |||
* Saddle Anaesthesia. | |||
* Weakness in Limbs and Saddle Anaesthesia. | |||
| | |||
* Urinary Retention, Followed by Incontinence. | |||
* Recent Loss of Bowel and Bladder control. | |||
* Saddle Anaesthesia. | |||
* Weakness in Limbs . | |||
* Age between 40-70. | |||
|- | |||
|Others : | |||
* Urinary Retention. | |||
* Incontinence | |||
In a primary care setting, malignancy is extremely rare. | * Age Between 40-70. | ||
|Others: | |||
* Recent Loss of Bowel Control. | |||
* Recent Loss of Bladder Control. | |||
* Urinary Retention, Followed by Incontinence. | |||
| | |||
|} | |||
=== Malignancy === | |||
The spinal cord may be compressed due to tumors occupying space within the vertebral canal. This may then affect the neural function of the spinal cord causing unremitting pain, muscle power and sensation alteration, sexual dysfunction, bladder/bowel dysfunction, and sleep disturbances.<ref name=":1">Cancer Research UK.org. 2020. Spinal Cord Tumours (Primary) | Cancer Research UK. [online] Available from: https://www.cancerresearchuk.org/about-cancer/brain-tumours/types/treatment-spinal-cord-tumours [last Accessed 20 May 2020].</ref> | |||
==== Epidemiology ==== | |||
Tumors are classified as primary, originating in the spine, and secondary, originating elsewhere in the body and spreading to the spine.<ref name=":1" /> Secondary tumors are much more prevalent than primary tumors. They occur in approximately 70% of cancer patients<ref>Ciftdemir M, Kaya M, Selcuk E, Yalniz E. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4757655/ Tumors of the spine.] World journal of orthopedics. 2016 Feb 18;7(2):109.</ref> whereas primary tumors occur in approximately 0.07% of healthy people.<ref name=":5">Schellinger KA, Propp JM, Villano JL, McCarthy BJ. [https://pubmed.ncbi.nlm.nih.gov/18084720/ Descriptive epidemiology of primary spinal cord tumors.] Journal of neuro-oncology. 2008 Apr 1;87(2):173-9.</ref> The most common types of primary tumors are meningiomas (29%), nerve-sheath tumors (24%), and ependymomas (23%).<ref name=":5" /> Secondary tumors can metastasize from many different areas of the body; most commonly they may spread from breast, lung, and prostate primary tumors.<ref>John Hopkins Medicine. 2020. Spinal Cancer And Spinal Tumors. [online] Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/spinal-cancer-and-spinal-tumors [Last Accessed 20 May 2020].</ref> | |||
In a primary care setting, malignancy is extremely rare. Table 4 highlights the incidence of primary diagnoses given that may result in low back pain within NHS primary care settings in the UK in 2018/19. 96,420,114 patients were seen in total.<ref name=":2" /> | |||
{| class="wikitable" | {| class="wikitable" | ||
|'''Condition''' | |'''Condition''' | ||
| Line 134: | Line 200: | ||
|0.00% | |0.00% | ||
|- | |- | ||
|Secondary malignant neoplasm of other unspecified parts of the | |Secondary malignant neoplasm of other unspecified parts of the nervous system | ||
|158 | |158 | ||
|0.00% | |0.00% | ||
| Line 143: | Line 209: | ||
|} | |} | ||
Table 4 - UK 2018/19 prevalence of Malignancy.<ref name=":1" /> | |||
Around 10-20% of patients diagnosed with spinal metastasis live for longer than two years after this diagnosis | ==== Prognosis ==== | ||
Around 10-20% of patients diagnosed with spinal metastasis live for longer than two years after this diagnosis.<ref>Delank KS, Wendtner C, Eich HT, Eysel P. [https://pubmed.ncbi.nlm.nih.gov/21311714/ The treatment of spinal metastases.] Deutsches Aerzteblatt International. 2011 Feb;108(5):71.</ref> Better prognoses and longer survival rates have been associated with earlier detection of the tumor.<ref>Ruckdeschel JC. [https://europepmc.org/article/med/15743153 Early detection and treatment of spinal cord compression.] Oncology. 2005 Jan 1;19(1).</ref> Therefore, it is important to screen patients with low back pain for red flags associated with spinal malignancy. | |||
==== Clinical Indicators ==== | |||
When assessing patients with low back pain, there are a number of ‘red flags’ which may increase suspicion of spinal malignancy. Studies have identified numerous clinical indicators of malignancy that should be screened for during the assessment of these patients.<ref name=":4" /> | |||
Studies accept that no single ‘red flag’ can be used in isolation to give a diagnosis of spinal malignancy. Instead, a combination may increase a clinician’s index of suspicion. The literature stated that patient-reported history of cancer, alongside low back pain, was identified as the most significant sign of spinal malignancy. Interestingly, Study reported that a past medical history of cancer, combined with unexplained weight loss, produced a specificity of 99.8%.<ref name=":3" /> The summary of findings from these studies is detailed in table 5 below. | |||
{| class="wikitable" | {| class="wikitable" | ||
|'''Indicator''' | |'''Indicator''' | ||
| Line 189: | Line 255: | ||
|99.8 | |99.8 | ||
|} | |} | ||
Table 5 - Clinical Indicators from Premkumar et al. (2018) & Tsiang et al. (2019).<ref name=":3" /><ref name=":4" /> | |||
=== Vertebral Fractures === | |||
[[Lumbar Spine Fracture|Vertebral fractures]] can be caused by direct or indirect trauma and are more likely to occur in patients with decreased bone density. Fractures may be classified as “stable” or if there is a risk of damage to the spinal cord “unstable”. A dorsal spine injury (vertebral arches, processes, and their ligaments) is always unstable and has a high probability of spinal cord injury.<ref name=":6">Amboss.com. 2020. ''Vertebral Fractures – Knowledge For Medical Students And Physicians''. [online] Available from: https://www.amboss.com/us/knowledge/Vertebral_fractures [Last Accessed 22 May 2020].</ref> | |||
==== Types of vertebral fractures ==== | |||
* Vertebral compression fracture | |||
* Loss of height of the vertebral body; due to trauma or pathological fracture | |||
* Progressive thoracic kyphotic deformity if multiple vertebrae are affected | |||
* Usually stable | |||
* Wedge fracture (subtype) | |||
* Burst fracture | |||
** Fracture of the vertebra in multiple locations | |||
** Result of compression trauma with severe axial loading | |||
** Possible displacement of bone fragments into the spinal canal | |||
* Fracture-dislocation | |||
** The fractured vertebra and disrupted ligaments | |||
The | ** Instability may cause spinal cord compression.<ref name=":6" /> | ||
==== Epidemiology ==== | |||
Of the 96,420,114 patients who were assessed in an NHS primary care setting in 2019, only 524 were diagnosed as having a Lumbar vertebral fracture. There were 498 diagnosed with a Fracture of other and unspecified parts of lumbar spine and pelvis, and 10 diagnosed with multiple fractures of lumbar spine and pelvis.<ref name=":2" /> | |||
{| class="wikitable" | |||
|'''Condition''' | |||
|'''Number of primary diagnoses''' | |||
|'''% of total cases''' | |||
|- | |||
|Low Back Pain | |||
|45,520 | |||
|0.04% | |||
|- | |||
|Fracture of lumbar vertebrae | |||
|524 | |||
|0.0% | |||
|- | |||
|Fracture of other and unspecified parts of lumbar spine and pelvis | |||
|498 | |||
|0.0% | |||
|- | |||
|Multiple fractures to lumbar spine and pelvis | |||
|10 | |||
|0.0% | |||
|} | |||
Table 6 - UK 2018/19 prevalence of Vertebrae Fractures | |||
'' | The actual incidence of vertebral fractures is likely much greater given the large number of vertebral fractures that go undetected. More than two-thirds of patients with Vertebral fractures are asymptomatic and are diagnosed incidentally.<ref>Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE. [https://pubmed.ncbi.nlm.nih.gov/15940375/ What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa?.] Journal of bone and mineral research. 2005 Jul;20(7):1216-22.</ref> Symptomatic patients may report the abrupt onset of pain with position changes, coughing, sneezing, or lifting.<ref name=":6" /> Physical examination findings are often 'normal' but may demonstrate kyphosis and midline spine tenderness.<ref name=":6" /> | ||
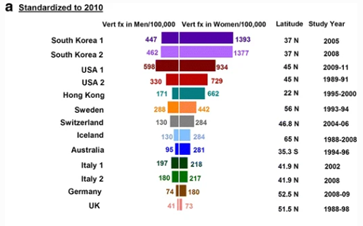
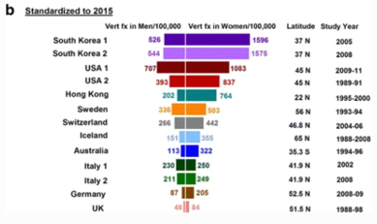
Ballane (2017) investigated the prevalence and incidence of vertebral fractures worldwide | Ballane (2017) investigated the prevalence and incidence of vertebral fractures worldwide. This study incorporated 62 articles of fair to good quality and comparable methods for vertebral fracture identification. Graphs A and B display age-standardised incidence rates in men and women combining hospitalized and ambulatory vertebral fractures, ranked by descending incidence. Standardisation to 2010 UN population (a) and 2015 UN population (b).<ref name=":8">Ballane G, Cauley JA, Luckey MM, Fuleihan GE. [https://europepmc.org/article/med/28168409 Worldwide prevalence and incidence of osteoporotic vertebral fractures.] Osteoporosis International. 2017 May 1;28(5):1531-42.</ref> | ||
Graphs A and B display age- | |||
{| class="wikitable" | {| class="wikitable" | ||
|[[File:Ballane 2017 Table inc countries.png|none|thumb|363x363px]] | |[[File:Ballane 2017 Table inc countries.png|none|thumb|363x363px]] | ||
| Line 209: | Line 311: | ||
|} | |} | ||
Figure 1 - Epidemiology of vertebrae fractures from Ballane, 2017.<ref name=":8" /> | |||
Rates partially depend on the definition of a vertebral fracture, clinical versus morphometric. The morphometric definition is not universal; at least seven methods have been used in different studies (included in Graphs A and B). Within the same method, varying decision thresholds (fracture grade or standard deviation from means) make the definition of vertebral fracture even more difficult. Different definitions include vertebral fracture include: | |||
* Morphometric method | |||
* McCloskey method | |||
* Eastell method | |||
* Genant Method | |||
* Davies method | |||
* Melton method | |||
* Denis Classification<ref name=":8" /> | |||
==== Diagnosis ==== | |||
Red flags for spinal fractures are signs and symptoms discovered by healthcare professionals during examinations, indicating potential issues within the spine. Ensuring the accuracy of these red flags is crucial because unreliable tests can result in incorrect diagnoses and treatment decisions. On one hand, inaccurate tests may lead to unnecessary imaging procedures (like X-rays or MRI scans) for individuals who don't have spinal fractures. These imaging methods can expose patients to radiation, incur additional costs, and cause unnecessary anxiety. On the other hand, failing to detect a spinal fracture can lead to delayed treatment and a reduced quality of life. Therefore, it is essential to identify the most precise red flags for screening spinal fractures.<ref name=":9">Han, C. S., Hancock, M. J., Downie, A., Jarvik, J. G., Koes, B. W., Machado, G. C., Verhagen, A. P., Williams, C. M., Chen, Q., & Maher, C. G. (2023). Red flags to screen for vertebral fracture in people presenting with low back pain. Cochrane Database of Systematic Reviews Review - Diagnostic. <nowiki>https://doi.org/10.1002/14651858.CD014461.pub2</nowiki></ref> | |||
In the primary care setting, between 1% and 5% of all patients who present with LBP will have a serious spinal pathology which requires further assessment and often specific treatment. <ref>Henschke N, Maher CG, Ostelo RW, de Vet HC, Macaskill P, Irwig L. [https://pubmed.ncbi.nlm.nih.gov/23450586/ Red flags to screen for malignancy in patients with low‐back pain.] Cochrane database of systematic reviews. 2013(2).</ref>The most common of these serious spinal pathologies which initially manifests as LBP is a vertebral fracture, followed by malignancy, infection, and inflammatory disease. The presence of a "red flag" should alert clinicians to the need for further examination and, in most cases, specific management. With respect to vertebral fractures, the typical “red flags” include >50 years of age, prolonged corticosteroid use, trauma, and Osteoporosis. High quality evidence investigating the efficacy of these “red flags” correctly identifying serious pathologies has been investigated in studies.<ref name=":3" /><ref name=":4" /> | |||
{| class="wikitable" | {| class="wikitable" | ||
| | |'''Indicator''' | ||
|'''Sensitivity (%)''' | |||
|'''Specificity (%)''' | |||
|Sensitivity | |- | ||
|Age of >50 years | |||
( | |74 | ||
|Specificity | |32.9 | ||
( | |||
| | |||
| | |||
| | |||
| | |||
|- | |- | ||
| | |Age of >70 years | ||
|3.9 | |||
|80 | |||
|. | |||
| | |||
|- | |- | ||
| | |Trauma | ||
| | |24.7 | ||
|88.6 | |||
| | |||
|- | |- | ||
| | |Combination 1: Trauma and age >50 years | ||
| | |14.8 | ||
|94.2 | |||
|. | |||
|- | |- | ||
| | |Combination 2: Trauma and age >70 years | ||
| | |5.2 | ||
| | |98.2 | ||
|. | |} | ||
Table 7 - Clinical Indicators from Premkumar et al. (2018).<ref name=":3" /> | |||
| | |||
| | {| class="wikitable" | ||
| | |'''Indicator''' | ||
| | |'''Sensitivity (%)''' | ||
|'''Specificity (%)''' | |||
|- | |- | ||
| | |Osteoporosis | ||
| | |41.5 | ||
|76.2 | |||
| | |||
|- | |- | ||
| | |Steroid use | ||
| | |28.3 | ||
|86.7 | |||
| | |||
|- | |- | ||
| | |Trauma | ||
| | |22.6 | ||
|93.8 | |||
| | |||
|} | |} | ||
Tsiang et al. (2019). | Table 8 - Clinical Indicators from Tsiang et al. (2019).<ref name=":4" /> | ||
The presence of both recent trauma and age of >50 years carries a 13.1% probability of a vertebral fracture in the setting of low back pain. The presence of both recent trauma and age of >70 years carries a 20.5% probability of vertebral fracture in the setting of low back pain.<ref name=":4" /> | |||
In fourteen studies<ref name=":9" /> that investigated various red flags used to identify spinal fractures, the four most effective red flags identified were corticosteroid use (e.g., medications that can weaken bones), age above 70 years, trauma (e.g., falls), and the presence of bruising or cuts. These findings have implications for different healthcare settings, from primary care to specialized hospital care. For instance, in primary healthcare settings, trauma was the most reliable indicator for screening unspecified and osteoporotic spinal fractures. Additionally, combining indicators like older age and gender worked well for detecting unspecified spinal fractures. In secondary healthcare settings, trauma remained a strong indicator for unspecified spinal fractures, while age was effective for osteoporotic spinal fractures. Combining age and trauma indicators was found to be most effective in secondary care for unspecified spinal fractures. Moreover, in tertiary care settings, the presence of contusions or abrasions proved to be the best indicator for screening spinal compression fractures. These findings underscore the importance of accurate red flags in the early detection and appropriate treatment of spinal fractures. | |||
==== Clinical implications ==== | |||
There is an agreement in the current literature the findings give rise to a weak recommendation that a combination of a small subset of red flags may be useful to screen for vertebral fracture. It should also be noted that many red flags have high false-positive rates; and if acted upon uncritically there would be consequences for the cost of management and outcomes of patients with LBP.<ref>Williams CM, Henschke N, Maher CG, van Tulder MW, Koes BW, Macaskill P, Irwig L. [https://pubmed.ncbi.nlm.nih.gov/23440831/ Red flags to screen for vertebral fracture in patients presenting with low‐back pain.] Cochrane Database of Systematic Reviews. 2013(1).</ref> | |||
=== Infection === | |||
Infections can often masquerade as MSK conditions of the lower back. Specifically, spinal infection (SI) is a term used to describe infectious diseases of the intervertebral discs, vertebral body, and paraspinal tissues. SI can develop through open trauma, surrounding areas that are infected, and through [[Bacterial Infections|bacteria]] entering the [[blood]] circulation spreading throughout the body. Evidence has shown that infections can cause redness of the skin, swelling, pain, heat, and raised [[Vital Signs|vital signs]]<ref>Nickerson EK, Sinha R. [https://academic.oup.com/bmb/article/117/1/121/1744712 Vertebral osteomyelitis in adults: an update.] British medical bulletin. 2016 Mar 1;117(1).</ref> | |||
==== Epidemiology ==== | |||
SI accounts for 2-7% of all MSK infections.<ref>Duarte RM, Vaccaro AR. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3843785/ Spinal infection: state of the art and management algorithm.] European Spine Journal. 2013 Dec 1;22(12):2787-99.</ref> A Japanese national figure database estimated the incidence of vertebral osteomyelitis to range from 5.3/100,000 to 7.4/100,000 population per year between 2007 and 2010.<ref>Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. [https://pubmed.ncbi.nlm.nih.gov/23533214/ Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database.] BMJ open. 2013 Jan 1;3(3).</ref> Postoperative SI is a common complication following spinal surgery. The incidence of SI post spinal surgery is reported to be between 0.1% to 6.7%.<ref>Dessy AM, Yuk FJ, Maniya AY, Connolly JG, Nathanson JT, Rasouli JJ, Choudhri TF. [https://www.cureus.com/articles/6040-reduced-surgical-site-infection-rates-following-spine-surgery-using-an-enhanced-prophylaxis-protocol Reduced surgical site infection rates following spine surgery using an enhanced prophylaxis protocol.] Cureus. 2017 Apr;9(4).</ref> | |||
''' | Similar to CES and malignancy, spinal infections are exceptionally rare within the primary care setting. This is highlighted in Table 9 which demonstrates the incidence of primary diagnoses of infections that can lead to low back pain within an NHS primary care setting in the UK in 2018/2019.<ref name=":2" /> | ||
{| class="wikitable" | |||
|'''Condition''' | |||
|'''Number of primary diagnoses''' | |||
|'''% of total cases''' | |||
|- | |||
|Low back pain | |||
|45,520 | |||
|0.00% | |||
|- | |||
|Osteomyelitis of vertebra | |||
|63 | |||
|0.00% | |||
|- | |||
|Osteomyelitis, unspecified | |||
|1558 | |||
|0.00% | |||
|- | |||
|Infection of intervertebral disc (pyogenic) | |||
|6 | |||
|0.00% | |||
|- | |||
|Discitis, unspecified | |||
|175 | |||
|0.00% | |||
|- | |||
|Infective myositis | |||
|65 | |||
|0.00% | |||
|- | |||
|Pyogenic arthritis, unspecified | |||
|359 | |||
|0.00% | |||
|- | |||
|Meningitis, unspecified | |||
|75 | |||
|0.00% | |||
|- | |||
|Infection following a procedure, not elsewhere classified | |||
|398 | |||
|0.00% | |||
|- | |||
|Intraspinal abscess and granuloma | |||
|13 | |||
|0.00% | |||
|} | |||
Table 9 - UK 2018/19 prevalence of Infection. | |||
==== Prognosis ==== | |||
Untreated infections can cause serious life-changing implications including severe neurological deficits. Numerous studies have reported successful treatment rates of 50% to 91% when administered antibiotics however, the mortality rate depends on the severity of co-morbidities and age.<ref>Kwon JW, Hyun SJ, Han SH, Kim KJ, Jahng TA. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5518432/ Pyogenic vertebral osteomyelitis: clinical features, diagnosis, and treatment.] Korean Journal of Spine. 2017 Jun;14(2):27.</ref> By recognizing the seriousness of implications if left untreated, it is of high importance that MSK physiotherapists are able to successfully screen patients for red flags associated with infection. | |||
==== Clinical Indicators ==== | |||
It is vital for clinicians to be aware of the ‘red flags’ when assessing patients presenting with low back pain. Awareness of the clinical signs and symptoms of serious pathologies such as infections, will allow the clinician to ask the right questions. This will help rule out serious and sinister pathologies and aid your clinical decision for the most appropriate pathway for further investigation.<ref name=":7" /> | |||
Large retrospective studies identified various ‘red flags’ for SI and evaluated their diagnostic accuracy in low back pain patients.<ref name=":3" /> These studies came to the same conclusions for SI as they did malignancy, fractures and CES. They concluded that due to the low sensitivity and specificity of ‘red flags’ it would be inappropriate to base your treatment decision on a singular ‘red flag’ in isolation. However, a combination should increase the clinicians index of suspicion and be used to probe further questioning or clinically reason further investigation. Premkumar et al (2018) provided further evidence to support the dismissal of using ‘red flags’ in isolation. Other results showed that negative responses to ’red flag’ questions did not decrease the likelihood of ‘red flag’ diagnoses. | |||
Due to the low prevalence of infection, it was difficult to assess the diagnostic accuracy of their associated ‘red flags’.<ref name=":4" /> In the Premkumar et al (2018) study, ‘red flag’ indicators for infection were based on published guidelines which included fever, chills, or sweating, recent infection, pain awakens from sleep and persistent sweating at night. Fever, chills, or sweating and recent infection all significantly increase the probability of SI. A summary of the findings from these studies are detailed in the table below. | |||
{| class="wikitable" | |||
|'''Indicator''' | |||
|'''Sensitivity''' (%) | |||
|'''Specificity (%)''' | |||
|- | |||
|Fever, chills, or sweating | |||
|11.7 | |||
|93.2 | |||
|- | |||
|Pain awakens from sleep | |||
|57.5 | |||
|41.8 | |||
|- | |||
|Persistent sweating at night | |||
|17.5 | |||
|86.1 | |||
|- | |||
|Recent infection | |||
|24.2 | |||
|97.4 | |||
|- | |||
|Night pain | |||
|37.5 | |||
|49.1 | |||
|- | |||
|Pain at rest | |||
|12.5 | |||
|69.7 | |||
|- | |||
|Fever | |||
|25.0 | |||
|97.6 | |||
|} | |||
Table 10 - Clinical Indicators from Premkumar et al. (2018)<ref name=":3" /> & Tsiang et al. (2019).<ref name=":4" /> | |||
Premkumar | |||
Yusuf et al (2019) conducted a descriptive review reporting on the characteristics of 2224 patients with SI. Interestingly, the most common clinical features were spinal pain (72%), fever (55%) and neurological dysfunction (33%). It was also found that SI was common in immunosuppressed patients due to other health conditions. The most common determinants were identified as diabetes (18%), IV drug use (9%) and recent surgery (6%). Although the diagnostic accuracy could not be tested, these findings within a large cohort are important to acknowledge to support your clinical reasoning and increase your index of suspicion.<ref>Yusuf M, Finucane L, Selfe J. [https://link.springer.com/article/10.1186/s12891-019-2949-6 Red flags for the early detection of spinal infection in back pain patients.] BMC Musculoskeletal Disorders. 2019 Dec 1;20(1):606.</ref> | |||
Ultimately, screening for ‘red flag’ indicators for SI does not change when assessing patients remotely. These screening questions are part of a subjective assessment therefore, can be conducted over a number of remote models of care. Finally, an accumulation of ‘red flags’ should lead the clinicians management of these patients and prompt further questioning and investigation when needed. | |||
== References == | |||
<references /> | |||
[[Category:Musculoskeletal/Orthopaedics]] | |||
[[Category:Lumbar Spine]] | |||
[[Category:Lumbar Spine - Assessment and Examination]] | |||
[[Category:Assessment]] | |||
[[Category:Nottingham University Spinal Rehabilitation Project]] | |||
Latest revision as of 15:28, 31 August 2023
Top Contributors - Tim Kirby, Zak Jackson, Manisha Shrestha, Nathan May, Kim Jackson, Adam Auckland, Shaimaa Eldib and Bruno Serra
Introduction[edit | edit source]
Red flags are clinical findings that are identified from a patient's medical history and the clinical exam and can increase the suspicion of serious pathology such as an infection, cancer, or a fracture[1]. The presence of red flags from a patient's subjective and objective assessment are thought to put them at a higher risk of serious pathology and warrant referral for further diagnostic testing.[2]
It is vital that practitioners are aware of these red flags as they form a key component of the assessment and management of low back pain whilst increasing patient safety.[3]
Red Flags and Low Back Pain[edit | edit source]
The role of physiotherapists as primary identifiers of red flags has grown owing to the spread of self‐referral services.[4] Physiotherapists often exist without any medical input or review.[5] [6]Therefore, there is a need to ensure that physiotherapists have a good understanding of individual red flags, understand their importance, and can ask these questions in a clear and unambiguous manner. Similarly, physiotherapists must have a clear understanding and agreed pathways of care dependent on these findings. Failure to do so raises issues around patient safety and professional reputation.[3]
One study by Feguson et al. (2015) aimed to investigate the red flags that are routinely recorded by physiotherapists[3]. This included which red flags do they consider to be most important, how would they define each red flag, and how they would ask each red‐flag question to a person with back pain. 98 physiotherapists responded to the survey, 84% worked exclusively in the National Health Service (NHS). They recorded that ‘Previous history of cancer, ‘saddle anesthesia, and difficulty with micturition were the red flags that raised suspicion of serious pathology the most. The physiotherapists involved in the study stated the following way to ask about red flags:
- History of cancer: an individual who has previously been diagnosed with cancer.
- Saddle anesthesia: Since your symptoms commenced, have you noticed any pins and needles or numbness around your back passage or genital area.
Finally, the limited consensus was found in how physiotherapists asked patients about red flags. However, one theme, in particular, emerged, which is the use of nebulous terminology - for example, the terms recent, weight loss, and prolonged period.[3]
COVID-19 and Remote Consultations[edit | edit source]
COVID-19 is an infectious respiratory disease, caused by the SARS-Cov-2 virus (Severe Acute Respiratory Syndrome Coronavirus Two) which spreads primarily through saliva and respiratory droplets when an infected person sneezes or coughs. Although the majority of people infected will only experience mild to moderate respiratory illness, the elderly and those with underlying health conditions are more likely to suffer from severe illness. As at November 2020 there have been over 61 million confirmed cases that have resulted in over 1.4 million deaths globally[7].
The reproduction number (R-value) is a method used to identify the disease's ability to spread meaning, this value represents the number of people that one infected person will pass the virus on to, on average.[8] R-value >1.0 means the disease will spread exponentially whereas, an R-value <1.0 means the disease will spread slowly and eventually die out.[9] The R-value is estimated to be between 1.4 and 2.5 which makes this disease significantly more contagious than the influenza virus.[10] This has forced healthcare services to rapidly implement alternative models of care to avoid face-to-face contact between patient and clinician.[11] Therefore, guidelines have been released for remote consultations based on current evidence evaluating its effectiveness.[12]
A systematic review (SR) compared the effectiveness of 'telerehabilitation' (defined by phone call and/or video consultations) and face-to-face consultations within a physiotherapy musculoskeletal (MSK) setting and result support its effectiveness.[13][14]
Qualitative studies have also provided insight into patient and clinician experiences during remote consultations. Positive feedback included time convenience, cost efficiency, easy to use technology, home environments, empowering self-management, and taking away the stigmatization of ‘hands-on’ therapy for treating low back pain.[15][16]
This evidence supports the use of remote consultations. Therefore, it is important to discuss how MSK physiotherapists can successfully identify and screen patients for red flags when presenting with low back pain during remote consultations, given the current global pandemic.
Low Back Pain Red Flags[edit | edit source]
Back pain is very common in the UK. It has a lifetime chance of occurrence of 59%.[17] The majority of back pain clears up quite quickly; however, back pain experienced along with ‘red flag’ symptoms may have a serious underlying cause.
This page discusses the clinical indicators for:
Cauda Equina[edit | edit source]
Cauda equina syndrome (CES) is a rare but potentially devastating neurological condition affecting the nerve roots at the lower end of the spinal cord known as the cauda equina (CE). The CE is responsible for the innervation of the lower limbs, control of the anal sphincter, regulation, and function of the bladder and distal bowel and sensation to the skin around the bottom and back passage.
'A patient presenting with acute back pain and/or leg pain with a suggestion of a disturbance of their bladder or bowel function and/or saddle sensory disturbance should be suspected of having a CES. Most of these patients will not have critical compression of the cauda equina. However, in the absence of reliably predictive symptoms and signs, there should be a low threshold for investigation with an emergency scan’.[18]
Epidemiology[edit | edit source]
There are many causes of CES with the most common being that of a lumbar spine disc herniation. It occurs most frequently between the ages of 31–50.[19] Cauda equina compression usually occurs at the level L4/5.[20] Although disc herniation is the most common mechanism, CES can be caused by any space-occupying lesion, such as spinal stenosis, tumor, cysts, infection, or bony ingress can narrow the spinal canal and cause compression of the cauda equina.[21]
Published estimates of the incidence for CES are fewer than one per 100 000 population.[22]
In a primary care setting, Table 1 highlights the incidence of diagnosed patients with CES in the UK 2018/19.[23]
| Condition | Number of primary diagnoses | % of total cases |
| Low Back Pain | 45,520 | 0.04% |
| Cauda Equina Syndrome | 170 | 0.0% |
| Malignant Neoplasm: Cauda Equina | 58 | 0.0% |
Prognosis[edit | edit source]
The prognosis for complete recovery is dependent upon many factors. The most important of these is the severity and duration of compression upon the damaged nerve(s). Generally, the longer the time before the intervention to remove the compression causing nerve damage, the greater the damage caused to the nerve(s). Similarly, Kennedy et al. (1999) describe the most important factor identified in a series of predictors for a favorable outcome in CES was an early diagnosis.[24] This highlights the importance of understanding and identifying red flags.
Clinical Indicators[edit | edit source]
The subjective history is the most important aspect of the examination, particularly early in the presentation of a patient with CES as the imperceptible and possible vague symptoms related to early CES need to be identified using clear and unmistakable methods of communication.[25] [26]
Premkumar et al. (2018) reported that the combination of recent loss of bladder control and recent loss of bowel control produced a specificity of 97.4%[27]. Both studies highlighted that while the specificity was generally high, all red flag questions had poor sensitivity when identifying their diagnoses of interest.
| Indicator | Sensitivity (%) | Specificity (%) |
| Recent loss of bladder control | 22.2 | 90.4 |
| Recent loss of bowel control | 13.9 | 95 |
| Combination: Recent loss of bladder and bowel control | 8.3 | 97.2 |
Table 2 - Clinical Indicators from Premkumar et al. (2018)[27].
| Indicator | Sensitivity (%) | Specificity (%) |
| Urinary retention | 30.3 | 96.2 |
| Incontinence | 50 | 86.5 |
| Saddle numbness | 0 | 94.7 |
| Weakness in limbs | 67.7 | 53.2 |
Table 3 - Clinical Indicators from Tsiang et al. (2019).[28]
During screening for red flags, Greenhalgh et al. (2015) in their qualitative investigation of patient's experience of CES found that one of the key problems in communication was the technical/medical language used by clinicians. For example, ‘saddle numbness’ to a patient is not clearly understood. The patient participants in the study emphasized the need for clinicians to use clear and some would say ‘explicit language’ that can be readily understood during a consultation.[29]
| Green
(Low Risk) |
Amber
(Intermediate Risk) |
Red
(High Risk) |
|---|---|---|
| Increased level of suspicion – Continue with assessment | Further increase in level of suspicion – Clinically Reason Between Option 1 and Option 2.
Option 1: Physiotherapy Appointment and/or GP referral Necessary Option 2: Urgent Physiotherapy Appointment and/or Urgent GP referral Necessary |
Further increase in level of suspicion for Cauda Equina Syndrome – Do Not continue with assessment Urgent A&E Referral Necessary |
Neurological:
|
Neurological
|
|
Others :
|
Others:
|
Malignancy[edit | edit source]
The spinal cord may be compressed due to tumors occupying space within the vertebral canal. This may then affect the neural function of the spinal cord causing unremitting pain, muscle power and sensation alteration, sexual dysfunction, bladder/bowel dysfunction, and sleep disturbances.[30]
Epidemiology[edit | edit source]
Tumors are classified as primary, originating in the spine, and secondary, originating elsewhere in the body and spreading to the spine.[30] Secondary tumors are much more prevalent than primary tumors. They occur in approximately 70% of cancer patients[31] whereas primary tumors occur in approximately 0.07% of healthy people.[32] The most common types of primary tumors are meningiomas (29%), nerve-sheath tumors (24%), and ependymomas (23%).[32] Secondary tumors can metastasize from many different areas of the body; most commonly they may spread from breast, lung, and prostate primary tumors.[33]
In a primary care setting, malignancy is extremely rare. Table 4 highlights the incidence of primary diagnoses given that may result in low back pain within NHS primary care settings in the UK in 2018/19. 96,420,114 patients were seen in total.[23]
| Condition | Number of primary diagnoses | % of total cases |
| Low back pain | 45,520 | 0.04% |
| Malignant neoplasm: Vertebral column | 136 | 0.00% |
| Malignant neoplasm: Connective and soft tissue of trunk | 85 | 0.00% |
| Malignant neoplasm: Spinal meninges | 1 | 0.00% |
| Malignant neoplasm: CNS unspecified | 233 | 0.00% |
| Malignant neoplasm of other and ill-defined sites: Lower limb | 74 | 0.00% |
| Secondary malignant neoplasm of other unspecified parts of the nervous system | 158 | 0.00% |
| Secondary malignant neoplasm of bone and bone marrow | 17,629 | 0.02% |
Table 4 - UK 2018/19 prevalence of Malignancy.[30]
Prognosis[edit | edit source]
Around 10-20% of patients diagnosed with spinal metastasis live for longer than two years after this diagnosis.[34] Better prognoses and longer survival rates have been associated with earlier detection of the tumor.[35] Therefore, it is important to screen patients with low back pain for red flags associated with spinal malignancy.
Clinical Indicators[edit | edit source]
When assessing patients with low back pain, there are a number of ‘red flags’ which may increase suspicion of spinal malignancy. Studies have identified numerous clinical indicators of malignancy that should be screened for during the assessment of these patients.[28]
Studies accept that no single ‘red flag’ can be used in isolation to give a diagnosis of spinal malignancy. Instead, a combination may increase a clinician’s index of suspicion. The literature stated that patient-reported history of cancer, alongside low back pain, was identified as the most significant sign of spinal malignancy. Interestingly, Study reported that a past medical history of cancer, combined with unexplained weight loss, produced a specificity of 99.8%.[27] The summary of findings from these studies is detailed in table 5 below.
| Indicator | Sensitivity (%) | Specificity (%) |
| Age >50 | 71.7 | 32.6 |
| Age >70 | 22.6 | 79.5 |
| Night pain | 54.2-55.4 | 41.8-49.6 |
| Unexplained weight loss | 8.2 | 95.6 |
| Pain at rest | 25 | 69.8 |
| Urinary retention | 4.2 | 95.8 |
| History of cancer | 32-75 | 78.7-95.6 |
| History of cancer + Unexplained weight loss | 2.5 | 99.8 |
Table 5 - Clinical Indicators from Premkumar et al. (2018) & Tsiang et al. (2019).[27][28]
Vertebral Fractures[edit | edit source]
Vertebral fractures can be caused by direct or indirect trauma and are more likely to occur in patients with decreased bone density. Fractures may be classified as “stable” or if there is a risk of damage to the spinal cord “unstable”. A dorsal spine injury (vertebral arches, processes, and their ligaments) is always unstable and has a high probability of spinal cord injury.[36]
Types of vertebral fractures[edit | edit source]
- Vertebral compression fracture
- Loss of height of the vertebral body; due to trauma or pathological fracture
- Progressive thoracic kyphotic deformity if multiple vertebrae are affected
- Usually stable
- Wedge fracture (subtype)
- Burst fracture
- Fracture of the vertebra in multiple locations
- Result of compression trauma with severe axial loading
- Possible displacement of bone fragments into the spinal canal
- Fracture-dislocation
- The fractured vertebra and disrupted ligaments
- Instability may cause spinal cord compression.[36]
Epidemiology[edit | edit source]
Of the 96,420,114 patients who were assessed in an NHS primary care setting in 2019, only 524 were diagnosed as having a Lumbar vertebral fracture. There were 498 diagnosed with a Fracture of other and unspecified parts of lumbar spine and pelvis, and 10 diagnosed with multiple fractures of lumbar spine and pelvis.[23]
| Condition | Number of primary diagnoses | % of total cases |
| Low Back Pain | 45,520 | 0.04% |
| Fracture of lumbar vertebrae | 524 | 0.0% |
| Fracture of other and unspecified parts of lumbar spine and pelvis | 498 | 0.0% |
| Multiple fractures to lumbar spine and pelvis | 10 | 0.0% |
Table 6 - UK 2018/19 prevalence of Vertebrae Fractures
The actual incidence of vertebral fractures is likely much greater given the large number of vertebral fractures that go undetected. More than two-thirds of patients with Vertebral fractures are asymptomatic and are diagnosed incidentally.[37] Symptomatic patients may report the abrupt onset of pain with position changes, coughing, sneezing, or lifting.[36] Physical examination findings are often 'normal' but may demonstrate kyphosis and midline spine tenderness.[36]
Ballane (2017) investigated the prevalence and incidence of vertebral fractures worldwide. This study incorporated 62 articles of fair to good quality and comparable methods for vertebral fracture identification. Graphs A and B display age-standardised incidence rates in men and women combining hospitalized and ambulatory vertebral fractures, ranked by descending incidence. Standardisation to 2010 UN population (a) and 2015 UN population (b).[38]
Figure 1 - Epidemiology of vertebrae fractures from Ballane, 2017.[38]
Rates partially depend on the definition of a vertebral fracture, clinical versus morphometric. The morphometric definition is not universal; at least seven methods have been used in different studies (included in Graphs A and B). Within the same method, varying decision thresholds (fracture grade or standard deviation from means) make the definition of vertebral fracture even more difficult. Different definitions include vertebral fracture include:
- Morphometric method
- McCloskey method
- Eastell method
- Genant Method
- Davies method
- Melton method
- Denis Classification[38]
Diagnosis[edit | edit source]
Red flags for spinal fractures are signs and symptoms discovered by healthcare professionals during examinations, indicating potential issues within the spine. Ensuring the accuracy of these red flags is crucial because unreliable tests can result in incorrect diagnoses and treatment decisions. On one hand, inaccurate tests may lead to unnecessary imaging procedures (like X-rays or MRI scans) for individuals who don't have spinal fractures. These imaging methods can expose patients to radiation, incur additional costs, and cause unnecessary anxiety. On the other hand, failing to detect a spinal fracture can lead to delayed treatment and a reduced quality of life. Therefore, it is essential to identify the most precise red flags for screening spinal fractures.[39]
In the primary care setting, between 1% and 5% of all patients who present with LBP will have a serious spinal pathology which requires further assessment and often specific treatment. [40]The most common of these serious spinal pathologies which initially manifests as LBP is a vertebral fracture, followed by malignancy, infection, and inflammatory disease. The presence of a "red flag" should alert clinicians to the need for further examination and, in most cases, specific management. With respect to vertebral fractures, the typical “red flags” include >50 years of age, prolonged corticosteroid use, trauma, and Osteoporosis. High quality evidence investigating the efficacy of these “red flags” correctly identifying serious pathologies has been investigated in studies.[27][28]
| Indicator | Sensitivity (%) | Specificity (%) |
| Age of >50 years | 74 | 32.9 |
| Age of >70 years | 3.9 | 80 |
| Trauma | 24.7 | 88.6 |
| Combination 1: Trauma and age >50 years | 14.8 | 94.2 |
| Combination 2: Trauma and age >70 years | 5.2 | 98.2 |
Table 7 - Clinical Indicators from Premkumar et al. (2018).[27]
| Indicator | Sensitivity (%) | Specificity (%) |
| Osteoporosis | 41.5 | 76.2 |
| Steroid use | 28.3 | 86.7 |
| Trauma | 22.6 | 93.8 |
Table 8 - Clinical Indicators from Tsiang et al. (2019).[28]
The presence of both recent trauma and age of >50 years carries a 13.1% probability of a vertebral fracture in the setting of low back pain. The presence of both recent trauma and age of >70 years carries a 20.5% probability of vertebral fracture in the setting of low back pain.[28]
In fourteen studies[39] that investigated various red flags used to identify spinal fractures, the four most effective red flags identified were corticosteroid use (e.g., medications that can weaken bones), age above 70 years, trauma (e.g., falls), and the presence of bruising or cuts. These findings have implications for different healthcare settings, from primary care to specialized hospital care. For instance, in primary healthcare settings, trauma was the most reliable indicator for screening unspecified and osteoporotic spinal fractures. Additionally, combining indicators like older age and gender worked well for detecting unspecified spinal fractures. In secondary healthcare settings, trauma remained a strong indicator for unspecified spinal fractures, while age was effective for osteoporotic spinal fractures. Combining age and trauma indicators was found to be most effective in secondary care for unspecified spinal fractures. Moreover, in tertiary care settings, the presence of contusions or abrasions proved to be the best indicator for screening spinal compression fractures. These findings underscore the importance of accurate red flags in the early detection and appropriate treatment of spinal fractures.
Clinical implications[edit | edit source]
There is an agreement in the current literature the findings give rise to a weak recommendation that a combination of a small subset of red flags may be useful to screen for vertebral fracture. It should also be noted that many red flags have high false-positive rates; and if acted upon uncritically there would be consequences for the cost of management and outcomes of patients with LBP.[41]
Infection[edit | edit source]
Infections can often masquerade as MSK conditions of the lower back. Specifically, spinal infection (SI) is a term used to describe infectious diseases of the intervertebral discs, vertebral body, and paraspinal tissues. SI can develop through open trauma, surrounding areas that are infected, and through bacteria entering the blood circulation spreading throughout the body. Evidence has shown that infections can cause redness of the skin, swelling, pain, heat, and raised vital signs[42]
Epidemiology[edit | edit source]
SI accounts for 2-7% of all MSK infections.[43] A Japanese national figure database estimated the incidence of vertebral osteomyelitis to range from 5.3/100,000 to 7.4/100,000 population per year between 2007 and 2010.[44] Postoperative SI is a common complication following spinal surgery. The incidence of SI post spinal surgery is reported to be between 0.1% to 6.7%.[45]
Similar to CES and malignancy, spinal infections are exceptionally rare within the primary care setting. This is highlighted in Table 9 which demonstrates the incidence of primary diagnoses of infections that can lead to low back pain within an NHS primary care setting in the UK in 2018/2019.[23]
| Condition | Number of primary diagnoses | % of total cases |
| Low back pain | 45,520 | 0.00% |
| Osteomyelitis of vertebra | 63 | 0.00% |
| Osteomyelitis, unspecified | 1558 | 0.00% |
| Infection of intervertebral disc (pyogenic) | 6 | 0.00% |
| Discitis, unspecified | 175 | 0.00% |
| Infective myositis | 65 | 0.00% |
| Pyogenic arthritis, unspecified | 359 | 0.00% |
| Meningitis, unspecified | 75 | 0.00% |
| Infection following a procedure, not elsewhere classified | 398 | 0.00% |
| Intraspinal abscess and granuloma | 13 | 0.00% |
Table 9 - UK 2018/19 prevalence of Infection.
Prognosis[edit | edit source]
Untreated infections can cause serious life-changing implications including severe neurological deficits. Numerous studies have reported successful treatment rates of 50% to 91% when administered antibiotics however, the mortality rate depends on the severity of co-morbidities and age.[46] By recognizing the seriousness of implications if left untreated, it is of high importance that MSK physiotherapists are able to successfully screen patients for red flags associated with infection.
Clinical Indicators[edit | edit source]
It is vital for clinicians to be aware of the ‘red flags’ when assessing patients presenting with low back pain. Awareness of the clinical signs and symptoms of serious pathologies such as infections, will allow the clinician to ask the right questions. This will help rule out serious and sinister pathologies and aid your clinical decision for the most appropriate pathway for further investigation.[1]
Large retrospective studies identified various ‘red flags’ for SI and evaluated their diagnostic accuracy in low back pain patients.[27] These studies came to the same conclusions for SI as they did malignancy, fractures and CES. They concluded that due to the low sensitivity and specificity of ‘red flags’ it would be inappropriate to base your treatment decision on a singular ‘red flag’ in isolation. However, a combination should increase the clinicians index of suspicion and be used to probe further questioning or clinically reason further investigation. Premkumar et al (2018) provided further evidence to support the dismissal of using ‘red flags’ in isolation. Other results showed that negative responses to ’red flag’ questions did not decrease the likelihood of ‘red flag’ diagnoses.
Due to the low prevalence of infection, it was difficult to assess the diagnostic accuracy of their associated ‘red flags’.[28] In the Premkumar et al (2018) study, ‘red flag’ indicators for infection were based on published guidelines which included fever, chills, or sweating, recent infection, pain awakens from sleep and persistent sweating at night. Fever, chills, or sweating and recent infection all significantly increase the probability of SI. A summary of the findings from these studies are detailed in the table below.
| Indicator | Sensitivity (%) | Specificity (%) |
| Fever, chills, or sweating | 11.7 | 93.2 |
| Pain awakens from sleep | 57.5 | 41.8 |
| Persistent sweating at night | 17.5 | 86.1 |
| Recent infection | 24.2 | 97.4 |
| Night pain | 37.5 | 49.1 |
| Pain at rest | 12.5 | 69.7 |
| Fever | 25.0 | 97.6 |
Table 10 - Clinical Indicators from Premkumar et al. (2018)[27] & Tsiang et al. (2019).[28]
Yusuf et al (2019) conducted a descriptive review reporting on the characteristics of 2224 patients with SI. Interestingly, the most common clinical features were spinal pain (72%), fever (55%) and neurological dysfunction (33%). It was also found that SI was common in immunosuppressed patients due to other health conditions. The most common determinants were identified as diabetes (18%), IV drug use (9%) and recent surgery (6%). Although the diagnostic accuracy could not be tested, these findings within a large cohort are important to acknowledge to support your clinical reasoning and increase your index of suspicion.[47]
Ultimately, screening for ‘red flag’ indicators for SI does not change when assessing patients remotely. These screening questions are part of a subjective assessment therefore, can be conducted over a number of remote models of care. Finally, an accumulation of ‘red flags’ should lead the clinicians management of these patients and prompt further questioning and investigation when needed.
References[edit | edit source]
- ↑ 1.0 1.1 Greenhalgh S, Finucane LM, Mercer C, Selfe J. Safety netting; best practice in the face of uncertainty. Musculoskeletal Science and Practice. 2020 May 12:102179.
- ↑ Delitto, A., George, S. and Godges. J, 2012. Low Back Pain Clinical Practice Guidelines Linked to the International Classification of Functioning, Disability, and Health from the Orthopaedic Section of the American Physical Therapy Association. Journal of orthopaedics and sports physical therapy. 42(4), pp. 57
- ↑ 3.0 3.1 3.2 3.3 Ferguson FC, Morison S, Ryan CG. Physiotherapists' understanding of red flags for back pain. Musculoskeletal care. 2015 Mar;13(1):42-50.
- ↑ Holdsworth LK, Webster VS, McFadyen AK, Scottish Physiotherapy Self Referral Study Group. Self-referral to physiotherapy: deprivation and geographical setting: is there a relationship? Results of a national trial. Physiotherapy. 2006 Mar 1;92(1):16-25.
- ↑ Kersten P, McPherson K, Lattimer V, George S, Breton A, Ellis B. Physiotherapy extended scope of practice–who is doing what and why?. Physiotherapy. 2007 Dec 1;93(4):235-42.
- ↑ McPherson K, Kersten P, George S, Lattimer V, Breton A, Ellis B, Kaur D, Frampton G. A systematic review of evidence about extended roles for allied health professionals. Journal of health services research & policy. 2006 Oct 1;11(4):240-7.
- ↑ World Health Organisation: Coronavirus Disease (COVID-19) Pandemic. [Last accessed 29 November 2020]
- ↑ Yi Y, Lagniton PN, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. International journal of biological sciences. 2020;16(10):1753.
- ↑ Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. International Journal of Infectious Diseases. 2020 Mar 18.
- ↑ Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of travel medicine. 2020 Mar 13.
- ↑ Greenhalgh T, Wherton J, Shaw S, Morrison C. Video consultations for covid-19.
- ↑ NHS.uk. 2020. GP Online And Video Consultations. [online] Available at: <https://www.nhs.uk/using-the-nhs/nhs-services/gps/gp-online-and-video-consultations/> [Accessed 20 April 2020].
- ↑ Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clinical rehabilitation. 2017 May;31(5):625-38.
- ↑ Shukla H, Nair SR, Thakker D. Role of telerehabilitation in patients following total knee arthroplasty: Evidence from a systematic literature review and meta-analysis. Journal of telemedicine and telecare. 2017 Feb;23(2):339-46.
- ↑ Hinman RS, Nelligan RK, Bennell KL, Delany C. “Sounds a bit crazy, but it was almost more personal:” a qualitative study of patient and clinician experiences of physical therapist–prescribed exercise for knee osteoarthritis via Skype. Arthritis care & research. 2017 Dec;69(12):1834-44.
- ↑ Synnott A, O’Keeffe M, Bunzli S, Dankaerts W, O'Sullivan P, O'Sullivan K. Physiotherapists may stigmatise or feel unprepared to treat people with low back pain and psychosocial factors that influence recovery: a systematic review. Journal of physiotherapy. 2015 Apr 1;61(2):68-76.
- ↑ GP online. 2008. Red flag symptoms: Back pain [Online]. Available from: https://www.gponline.com/red-flag-symptoms-back-pain/musculoskeletal-disorders/musculoskeletal-disorders/article/798743 [Accessed 20/05/20]
- ↑ Germon T, Ahuja S, Casey ATH, Todd NV, Rai A. British Association of Spine Surgeons standards of care for cauda equina syndrome. Spine J. 2015 Mar 2;15(3 Suppl):S2-S4. doi: 10.1016/j.spinee.2015.01.006. PMID: 25708139.
- ↑ Fuso FA, Dias AL, Letaif OB, Cristante AF, Marcon RM, Barros Filho TE. Epidemiological study of cauda equina syndrome. Acta ortopedica brasileira. 2013 Jun;21(3):159-62.
- ↑ Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009 Nov;90(11):1964-8. doi: 10.1016/j.apmr.2009.03.021. PMID: 19887225.
- ↑ Greenhalgh S, Finucane L, Mercer C, Selfe J. Assessment and management of cauda equina syndrome. Musculoskelet Sci Pract. 2018 Oct;37:69-74. doi: 10.1016/j.msksp.2018.06.002. Epub 2018 Jun 7. PMID: 29935940.
- ↑ Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2007 Apr;35(4):529-31.
- ↑ 23.0 23.1 23.2 23.3 Barnes, M., 2019. NHS Digital, Hospital Episode Statistics For England. Outpatient Statistics, 2018 - 2019.. Primary Diagnosis by Attendance Type. NHS Digital.
- ↑ Kennedy JG, Soffe KE, McGrath A, Stephens MM, Walsh MG, McManus F. Predictors of outcome in cauda equina syndrome. European Spine Journal. 1999 Aug 1;8(4):317-22.
- ↑ Bin MA, Hong WU, Jia LS, Wen YU, Shi GD, Shi JG. Cauda equina syndrome: a review of clinical progress. Chinese medical journal. 2009 May 1;122(10):1214-22.
- ↑ Sun JC, Xu T, Chen KF, Qian W, Liu K, Shi JG, Yuan W, Jia LS. Assessment of cauda equina syndrome progression pattern to improve diagnosis. Spine. 2014 Apr 1;39(7):596-602.
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 27.7 Premkumar A, Godfrey W, Gottschalk MB, Boden SD. Red flags for low Back pain are not always really red: a prospective evaluation of the clinical utility of commonly used screening questions for low Back pain. JBJS. 2018 Mar 7;100(5):368-74.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 Tsiang JT, Kinzy TG, Thompson N, Tanenbaum JE, Thakore NL, Khalaf T, Katzan IL. Sensitivity and specificity of patient-entered red flags for lower back pain. The Spine Journal. 2019 Feb 1;19(2):293-300.
- ↑ Greenhalgh S, Truman C, Webster V, Selfe J. An investigation into the patient experience of Cauda Equina Syndrome: A qualitative study. Physiotherapy Practice and Research. 2015 Jan 1;36(1):23-31.
- ↑ 30.0 30.1 30.2 Cancer Research UK.org. 2020. Spinal Cord Tumours (Primary) | Cancer Research UK. [online] Available from: https://www.cancerresearchuk.org/about-cancer/brain-tumours/types/treatment-spinal-cord-tumours [last Accessed 20 May 2020].
- ↑ Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World journal of orthopedics. 2016 Feb 18;7(2):109.
- ↑ 32.0 32.1 Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. Journal of neuro-oncology. 2008 Apr 1;87(2):173-9.
- ↑ John Hopkins Medicine. 2020. Spinal Cancer And Spinal Tumors. [online] Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/spinal-cancer-and-spinal-tumors [Last Accessed 20 May 2020].
- ↑ Delank KS, Wendtner C, Eich HT, Eysel P. The treatment of spinal metastases. Deutsches Aerzteblatt International. 2011 Feb;108(5):71.
- ↑ Ruckdeschel JC. Early detection and treatment of spinal cord compression. Oncology. 2005 Jan 1;19(1).
- ↑ 36.0 36.1 36.2 36.3 Amboss.com. 2020. Vertebral Fractures – Knowledge For Medical Students And Physicians. [online] Available from: https://www.amboss.com/us/knowledge/Vertebral_fractures [Last Accessed 22 May 2020].
- ↑ Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, Black DM, Ensrud KE. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa?. Journal of bone and mineral research. 2005 Jul;20(7):1216-22.
- ↑ 38.0 38.1 38.2 Ballane G, Cauley JA, Luckey MM, Fuleihan GE. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporosis International. 2017 May 1;28(5):1531-42.
- ↑ 39.0 39.1 Han, C. S., Hancock, M. J., Downie, A., Jarvik, J. G., Koes, B. W., Machado, G. C., Verhagen, A. P., Williams, C. M., Chen, Q., & Maher, C. G. (2023). Red flags to screen for vertebral fracture in people presenting with low back pain. Cochrane Database of Systematic Reviews Review - Diagnostic. https://doi.org/10.1002/14651858.CD014461.pub2
- ↑ Henschke N, Maher CG, Ostelo RW, de Vet HC, Macaskill P, Irwig L. Red flags to screen for malignancy in patients with low‐back pain. Cochrane database of systematic reviews. 2013(2).
- ↑ Williams CM, Henschke N, Maher CG, van Tulder MW, Koes BW, Macaskill P, Irwig L. Red flags to screen for vertebral fracture in patients presenting with low‐back pain. Cochrane Database of Systematic Reviews. 2013(1).
- ↑ Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. British medical bulletin. 2016 Mar 1;117(1).
- ↑ Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. European Spine Journal. 2013 Dec 1;22(12):2787-99.
- ↑ Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ open. 2013 Jan 1;3(3).
- ↑ Dessy AM, Yuk FJ, Maniya AY, Connolly JG, Nathanson JT, Rasouli JJ, Choudhri TF. Reduced surgical site infection rates following spine surgery using an enhanced prophylaxis protocol. Cureus. 2017 Apr;9(4).
- ↑ Kwon JW, Hyun SJ, Han SH, Kim KJ, Jahng TA. Pyogenic vertebral osteomyelitis: clinical features, diagnosis, and treatment. Korean Journal of Spine. 2017 Jun;14(2):27.
- ↑ Yusuf M, Finucane L, Selfe J. Red flags for the early detection of spinal infection in back pain patients. BMC Musculoskeletal Disorders. 2019 Dec 1;20(1):606.