Instrumented Gait Analysis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 206: | Line 206: | ||
EMG data normalization methods, advantages and limitations<ref name=":6" /><ref name=":7" /><ref name=":0" />: | EMG data normalization methods, advantages and limitations<ref name=":6" /><ref name=":7" /><ref name=":0" />: | ||
{| border="1" cellpadding="1" cellspacing="1" | |||
|- | |||
! scope="row"| Method for normalization | |||
! scope="col" | Advantages | |||
! scope="col" | Limitations | |||
|- | |||
! scope="row" |Maximal voluntary isometric contraction | |||
| Frequently used in studies, limits the variability of EMG data, provides information about muscle activation | |||
| Not all patients are able to produce Maximal voluntary isometric contraction due to fatigue, participation from the patients (pediatrics), repetition in time | |||
|- | |||
! scope="row" | Sub maximal voluntary contraction (% of maximal voluntary contraction) | |||
|Useful in case the patient can’t produce maximal voluntary contraction, easier to maintain | |||
|Still needs to maximal contraction values to normalize from | |||
|- | |||
! scope="row" | Instrumentally evoked contraction (stimulation) | |||
|Activates all motor units to reach “tetanus” level without any participation of the patient | |||
| Discomfort, doesn’t give information about patient’s activation pattern | |||
|- | |||
! scope="row" | Reference contraction: one movement is repeated by the subject and the middle period is taken as the reference contraction | |||
| Provides a stable reference value, closer to lower activation levels for muscles which do not physiologically contract at the maximal level (ex: tibial posterior during gait) | |||
| Discomfort, doesn’t give information about patient’s activation pattern | |||
|- | |||
! scope="row" | Mean or peak value of EMG set during a full gait cycle | |||
| Limits the variability of EMG data, reduces inter participants difference, allow to detect normal/abnormal muscle activation during gait | |||
| Expose data to confoundable variable: force-velocity relationship and change of muscle mass located under the electrode | |||
Modifies EMG data amplitude | |||
|- | |||
! scope="row" |Muscle activity at rest | |||
|Interesting for patients with neurological disorders | |||
| No information on the muscle and possible confusion between various muscle groups. | |||
|- | |||
|} | |||
'''<u>Interpreting EMG Data:</u>'''Once processed and normalized, clinicians can make various interpretations from EMG data such as the motor unit firing trains, which refer to the moments when the motor unit fires (MUAP): it informs clinicians about the activation/nonactivation of the muscle. In addition, the recruitment / de-recruitment threshold of a motor unit which can be interpreted as an indication of the force level at which the motor unit starts and stops firing during a contraction. Within the same muscle, different thresholds can be observed and be unlocked as the need for force increases. The shape of MUAP provides information about the morphology of the motor unit and the state of the motor fibers. EMG data can also be used to calculate the motor unit’s mean firing rate (number of pulses per unit of time) and the synchronization of a motor unit with another<ref name=":6" /><ref name=":4" />. | '''<u>Interpreting EMG Data:</u>'''Once processed and normalized, clinicians can make various interpretations from EMG data such as the motor unit firing trains, which refer to the moments when the motor unit fires (MUAP): it informs clinicians about the activation/nonactivation of the muscle. In addition, the recruitment / de-recruitment threshold of a motor unit which can be interpreted as an indication of the force level at which the motor unit starts and stops firing during a contraction. Within the same muscle, different thresholds can be observed and be unlocked as the need for force increases. The shape of MUAP provides information about the morphology of the motor unit and the state of the motor fibers. EMG data can also be used to calculate the motor unit’s mean firing rate (number of pulses per unit of time) and the synchronization of a motor unit with another<ref name=":6" /><ref name=":4" />. | ||
Revision as of 11:41, 10 June 2020
Original Editor - Mariam Hashem
Top Contributors - Mariam Hashem, Jess Bell, Aminat Abolade, Kim Jackson, Tony Lowe and Tarina van der Stockt
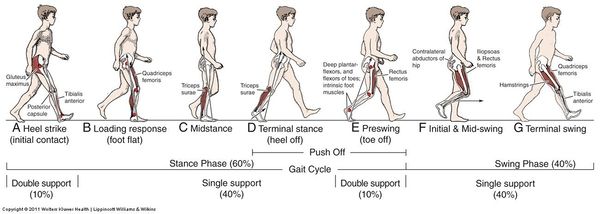
What is Normal Gait?[edit | edit source]
Normal gait is a range of typical gait patterns, found in a healthy population, presenting similar characteristics.[1] People present a certain degree of variability that is called inter-subject variability and is due to differences in age, gender, muscular strength and anatomical differences[2].
For example. if we are to assess the gait of an 80-year-old man and a 20-year-old woman, both without any pathologies affecting gait. They might present completely different gait patterns. However, they will both be considered physiological or normal because their gait patterns will be within the range of normality corresponding to their own population. So when assessing the gait of a patient in physiotherapy, the idea is to confront the gait parameters we find with our own patient against the range of normality established for the corresponding population[3][1].
Visual Gait Analysis[edit | edit source]
A commonly used method by physiotherapists to investigate gait problems with their patients using a smartphone or any video recording instrument.
Pros:
- Quick and easy method
- Allows peer-reviewing, by showing your colleagues the videos and discussing the presented case
- Reproducible, by taking multiple videos you can track your patient's progression
- Allows observing the gait from multiple angles to detect deviations in multiple plans
Cons:
- The reliability is not very high and it depends on the clinical experience[4][1]
- It doesn't allow observing high-velocity events, force in moments during walking.
- Subjective, It depends on the observer therefore prone to error[3]
Instrumented Gait Analysis[edit | edit source]
Refers to the collection of quantitative data of the gait cycle, such as videography, kinematics, kinetics, oxygen consumption, and electromyography[6][7].
Literature suggests that instrumented gait analysis is a valuable tool in clinical practice for the diagnosis, the assessment, and the management of patients affected by conditions altering the gait. In the research field[8], instrumented gait analysis is often used to evaluate the effectiveness of treatments such as foot orthoses and to explore the consequences of pathologies related to gait, like Rheumatoid Arthritis.[3]
Uses:
- To apply in clinical practice
- To get familiar with the terminology that is often used in research publications and scientific articles[3]
For the purpose of providing a non-biased interpretation of the results, it is important to acknowledge all sources of data’s variability. Once these sources of variability are acknowledged or considered negligible, results can be interpreted with higher confidence[3].
- Inter-subject variability: refers to the difference of gait parameters obtained with different subjects. It can be due to anatomical differences, or difference in muscular strength[2] These differences often result in variation of spontaneous walking speed, an indicator of one’s own 2 adjustment to reach the lowest energy expenditure while walking[1].
- Within-subject variability: refers to the possibility of obtaining slightly different gait parameters on two different trials with the same person[7]. This can be caused by small changes in one person's gait from one trial to another due to, for example, stress or apprehension or the desire of the patient to do his best performance. Also, it can be caused by the measurement techniques which give slightly different results under the same conditions[3]
In gait analysis, reliability refers to the consistency of the results across multiple repetitions (within-subject or absolute reliability) and the consistency of the results within a cohort of subjects (inter-subject or relative reliability)[9]. It is a key feature for making correct interpretation of the results and generalizes them in other conditions than the ones of the experiment[10]. For example, information regarding the reliability of the measurements should be clearly exposed by authors publishing a randomized controlled trial using instrumented gait analysis as the method of assessment[3].
Electromyography[edit | edit source]
Electromyography (EMG) refers to the measurement of the electrical activity of a contracting muscle and by measuring the motor unit potential action(MUAP) [1]. A motor unit is a group of muscular fibers innervated by the same motoneuron. The MUAP refers to the characteristic shape of the depolarization voltage of the motor unit. The signal recorded by EMG is a summation of the electrical activity from a number of MUAP that are simultaneously activated during a muscle contraction[11]. Muscle activation patterns are analyzed through the electrical signals (EMG) associated with muscular fiber contraction, that can be recorded noninvasively through surface electrodes attached to the skin over muscle bellies[12]. Electromyography does not give information about[13]:
- The type of contraction (concentric, eccentric or isometric)
- The force produced by the muscle during this contraction.
However, EMG facilitates the understanding of the onset of contractions. This is made by recording the signal's magnitude and frequency of the muscles and refers to the amount of electrical activity during the contraction and the range of firing rates of the bar units that are being recorded[3].
Methods used to record muscle activity:
- Invasive methods using fine wire and needle. They are not appropriate for gait analysis.
- Surface EMG, which is the most commonly used method to record muscle activity during gait. Surface EMG is a non-invasive method, so it's really well tolerated by patients. It can be wireless, and there are various protocols that describe the correct placement of the electrodes to record the muscle activity of muscle groups. So it's a little less precise than other methods, but usually plenty sufficient for gait analysis.
Advantages and limitations of EMG techniques[3][2]:
| Method | Advantages | Limitation |
|---|---|---|
| Surface EMG (sEMG) | Non-invasive, operator doesn’t need training to place electrodes, highly tolerated, wireless / telemetry, possibility to use a protocol for electrode placements, various designs, allows prolonged recordings from multiple sites, most frequently reported in the literature, low-cost. | Records superficial signal records a group of motor units, movement of electrodes in relation to the bony references, inadequate for overweight patients, cross talk, noise, artefacts. |
| Intramuscular myo graphy (fine wire and needle) | Allow recording deep muscles and single motor unit, signal is stable, higher amplitude and frequency recording, less sensitive that sEMG to noise. | Invasive, damages muscle fibers, risk of infection, can modify patient’s movement because of discomfort/pain, mechanical artifacts, cables, requires operator training, wire can break. |
Before undertaking surface EMG, clinicians need to place electrodes on the patient’s skin surface. For this purpose, palpation combined with “testing” (active muscle contraction) is helpful to identify as precisely as possible the location of muscle’s bodies (Ewins & Collins, 2014)[2]. In order to favor reproducibility of measurements, Hermens et al published recommendations for the “surface EMG for a non-invasive assessment of muscles”[14]
In order to favor reproducibility of measurements, Hermens et al., (2000) published recommendations for the “surface EMG for a non-invasive assessment of muscles” (SENIAM). Below is a summary of the recommendations from the SENIAM (Hermens et al., 2000)[3]:
| Recommendation | Description | |
|---|---|---|
| Electrode shape and size | Researchers undertaking sEMG should report the type, manufacture and shape of the electrodes. The size of the electrode (size of the conductive area of the electrode) should not exceed 10mm. | |
| Inter electrode distance | Bipolar sEMG electrodes should respect an inter-electrode distance of 20mm. For small muscles, the inter-electrode distance should not exceed one quarter of the muscle fiber length. | |
| Electrode material | The electrode material in contact with the skin should provide good contact, low impedance and stable behavior in time. | Vertical |
| Sensor construction | Authors recommend fixed electrode distances using light materials. Cables motion should be limited with tape for example in order to reduce mechanical artefacts. | |
| Sensor placement procedure | The procedure contains 6 steps for which recommendations are the following:
1. Selection of EMG sensor: adapted electrode (shape, type, size) depending of the muscle studied. 2. Skin preparation: shave the area, clean the skin with alcohol and keep the electrode contact area dry. 3. Positioning the patient for manual muscle testing. 4. Determining sensor location: sensors should be placed in a stable location. The ideal location is defined in relation to the longitudinal / transversal location of the sensor on the muscle. For the longitudinal location: halfway between distal motor endplate zone and the distal tendon for longitudinal. For the transversal location: away from the edge of the muscle belly divisions and as close as possible as the middle of the muscle belly. 5. Sensor placement and fixation: electrodes orientation should be placed parallel to the muscle fibers. Electrodes should be stabilized using tape or elastic band in order to limit mechanical artefacts. 6. Testing of the connection: subject’s produce a voluntary contraction and clinician can observe in real time that the signal is recorded |
Despite being the most commonly used technique, sEMG presents certain limitations. The technique is based on the measurement of voltage difference between two electrodes and the use of a grounding electrode (somewhere else on the body)[11]The electrical signal that reaches the electrode on the skin surface is usually small because it has been attenuated by the layers of fascia, fat and skin underlying (noise effect). Therefore, the signal is amplified (1000 to 10 000 times) close to the electrodes and picked-up by electrodes as the sum of the muscle action potentials from many motor units within the most superficial muscle(s)[15]EMG signal is influenced by intrinsic and extrinsic variables which can affect the data recorded and therefore the reliability of interpretations[3].
Variables influencing EMG signal[16][3]:
| Variables | Cause | Consequence / Possible way to limit variable |
|---|---|---|
| Intrinsic | Tissue characteristic (type of muscle, motor unit size, presence of fat, skin temperature, etc.) | Affects electrical conductivity |
| Crosstalk (undesired recording of the activity of adjacent muscles) | Interference with EMG signal.
Selecting a group rather than a particular muscle | |
| Mechanical artefact (change in the electrical baseline happening when subject moves) | Contaminate EMG signal with “fake” activity. | |
| Internal noise (thermal noise) | Contaminate EMG signal with “fake” activity.
Locating amplifiers very close to the recording electrodes, therefore reducing the length of wire | |
| Skin (preparation, thickness, sweat, etc.) | Impedance of the signal and signal amplitude | |
| Extrinsic | Changes in muscle geometry between muscle belly and electrode site | Modifies EMG reading |
| External noise (amplifiers) | Contaminate EMG signal with “fake” activity | |
| Electrodes (placement regarding the motor point, muscle fiber orientation, etc.) | Affects conduction velocity | |
| Environmental conditions (temperature, humidity, interferences: electromagnetic disturbances coming from surrounding electrical equipment, etc.) | Signal amplitude | |
| Contraction conditions (type of contraction, muscle length, etc.) | Signal amplitude | |
| Signal processing (filters, etc.) | Signal amplitude |
Processing EMG Data: The first signal obtained after recording is called “Raw EMG” from which it is possible to see if a muscle is active or not. “Raw” EMG presents a wide range of frequency (from 5 to 500 Hz), artifacts, many data points, and positive and negative values which make the interpretation of the results difficult. In order to address this challenge, different techniques of data processing are available such as filtering, Root mean square, smoothing, Rectification, integration, and amplitude[7].
Signal processing methods, advantages and limitations[16][7][3]:
| Methods | Description | Advantages | Limitations |
|---|---|---|---|
| Filtering | Reduces/eliminates unwanted signal associated with noise/artefact | Makes data interpretation easier | Not acknowledging all the data can bias the interpretation of the results, not all studies use the same filters
Selecting the correct filter is important to no lose valuable data |
| Root mean square | Squaring all the values, taking the mean and finally the square roots | Removes negatives values
Linked with electrical power Preferred method in literature |
Details on the data use for the mean calculation should be provided |
| Smoothing | Average calculated for a specific window | Reproducible | Data details are lost because of the averaging |
| Rectification | Reflection of negative values to convert data in “all positive” | Makes statistical manipulation and reading easier | |
| Integration | Mathematical calculation of the “area” under the EMG graphic function. | Useful for relatively long duration segments | |
| Amplitude | Peak amplitude average of first 10 highest peaks | Useful for comparison analysis
True mathematical integral under EMG amplitude |
Does not reflect force production or effect of force on the joint |
Normalization of Data: EMG data is often normalized (rescaled to a reference value) using various techniques in order to facilitate interpretation, for example when comparing EMG recordings between different days, subjects or muscles. Authors suggest that the technique for normalization should be chosen according to the possibilities of the patients, for example in producing a “true” maximal voluntary isometric contraction[16]
EMG data normalization methods, advantages and limitations[16][15][3]:
| Method for normalization | Advantages | Limitations |
|---|---|---|
| Maximal voluntary isometric contraction | Frequently used in studies, limits the variability of EMG data, provides information about muscle activation | Not all patients are able to produce Maximal voluntary isometric contraction due to fatigue, participation from the patients (pediatrics), repetition in time |
| Sub maximal voluntary contraction (% of maximal voluntary contraction) | Useful in case the patient can’t produce maximal voluntary contraction, easier to maintain | Still needs to maximal contraction values to normalize from |
| Instrumentally evoked contraction (stimulation) | Activates all motor units to reach “tetanus” level without any participation of the patient | Discomfort, doesn’t give information about patient’s activation pattern |
| Reference contraction: one movement is repeated by the subject and the middle period is taken as the reference contraction | Provides a stable reference value, closer to lower activation levels for muscles which do not physiologically contract at the maximal level (ex: tibial posterior during gait) | Discomfort, doesn’t give information about patient’s activation pattern |
| Mean or peak value of EMG set during a full gait cycle | Limits the variability of EMG data, reduces inter participants difference, allow to detect normal/abnormal muscle activation during gait | Expose data to confoundable variable: force-velocity relationship and change of muscle mass located under the electrode
Modifies EMG data amplitude |
| Muscle activity at rest | Interesting for patients with neurological disorders | No information on the muscle and possible confusion between various muscle groups. |
Interpreting EMG Data:Once processed and normalized, clinicians can make various interpretations from EMG data such as the motor unit firing trains, which refer to the moments when the motor unit fires (MUAP): it informs clinicians about the activation/nonactivation of the muscle. In addition, the recruitment / de-recruitment threshold of a motor unit which can be interpreted as an indication of the force level at which the motor unit starts and stops firing during a contraction. Within the same muscle, different thresholds can be observed and be unlocked as the need for force increases. The shape of MUAP provides information about the morphology of the motor unit and the state of the motor fibers. EMG data can also be used to calculate the motor unit’s mean firing rate (number of pulses per unit of time) and the synchronization of a motor unit with another[16][11].
Reliability of surface EMG: EMG data, such as other types of data collected during gait analysis is quantitative and can therefore be evaluated in term osf reliability using statistical manipulation[17]. Smith[16] suggest that the reliability of EMG data is an important consideration as the quality of the results depends directly on it. For example, inter-participant reliability is important for making valid comparisons between subjects from a control and intervention group, as it ensures that the differences observed are due to the intervention[9]. Different features can be assessed for reliability such as the way the muscle activity is produced (evoked contraction / natural contraction), the interval between measurements, the comparability within-subjects, and environmental conditions, the material used for recording, . For example, Kollmitzer et al [18] suggest that long-term intervals for measurement (6 weeks) were correlated with lower reliability; whereas high reliability could be obtained in short interval measurements (within 90min). These results suggest that EMG could be a valuable technique to undertake an evaluation of direct effect interventions such as orthotics for example (with/without trial). However, these results are questioning the quality of evidence brought to clinicians by EMG measurements, as they are often used to assess the effectiveness of training and rehabilitation programs showing a positive impact on patients after a long period of time (4 weeks and more).
Regarding normalization, Smith[16] suggests MVIC has the best inter-participant reliability, followed by peak dynamic amplitude and single leg stance measurements. In addition, the choices made by researchers / clinicians regarding data collection depend on the abilities of the patient to comply with rigorous research protocol, but they should be enounced explicitly so readers can be critical on the reliability of the results[1].
Kinematics[edit | edit source]
Kinematics refers to the characterization of the movement of the different body parts, usually the legs. We also use a representation in segments. such as foot, knee, hips, and trunk.
For the purpose of recording gait kinematics, we place different markers on the patient's body. These markers are reflective, meaning that the position and the timing of walking are recorded by different cameras that are placed all over the laboratory. Then the position of the markers is integrated into a computer, which allows a 3D representation of the patient's walking, as well as the velocity and the acceleration of different body parts.
The kinematic data provides realtime precise information on the position of the joints during gait. For example, patients with Rheumatoid Arthritis have shown flat feet, excessive dorsal flexion of the ankle, as well as increased valgus of the ankle in the frontal plane during the stance phase. With kinematic data, It is also possible to obtain a level of precision up to one degree for a joint's range of motion or one millimeter for example, like a marker placed on a bony reference.
kinetic data[edit | edit source]
This refers to the characterizations of forces and moments applied to the patient while walking. For example, the ground force reaction or plantar pressure.
Kinetic data can be recorded with different methods:
The forced platform, which is usually used to characterize the different components of the ground force reaction. Special pressure sensitive insoles that can be used to calculate plantar pressures. For each studied reaction, it is possible to isolate values that have a clinical meaning. For example, when recording plantar pressure, it is possible to isolate values like the peak pressure or the time during the peak pressure is applied. The literature has shown an association of the development of ulcers, with people with certain values that are quite high.
The integration of kinetic, kinematic data as well as anthropometric values using a computer model that calculates the different moments and power produced and applied during gait. For example, researchers have been able to demonstrate that below-knee amputees using a ..... foot with a classic prosthetic leg, have a decrease in 50% in ankle power generation at the toe-off moment in the walking cycle.
Energy Expenditure[edit | edit source]
And the literature suggests that the best way to calculate energy expenditure is by calculating the oxygen uptake and carbon dioxide production as they reflect the patient's metabolism chemistry.
To obtain this, the patient wears a face mask, usually very uncomfortable, and they are asked to walk around while wearing the mask, The consumed oxygen and produced carbon dioxide are then calculated.
Conclusion[edit | edit source]
Instrumented gait analysis can be a valuable tool to quantify gait parameters. It is true that it is mostly applied in the research field to date, but we can hope, with the simplification of technology, it will be more available to a large number of physiotherapists in their day to day practice. As an alternative to instrumented gait analysis, visual analysis supported by video recording is a valuable tool to assess our patient's gait. And even better if we can include a validated tool like the gait deviation index that allows more reproducibility and are less observer-dependent.
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Levine D, Richards J, Whittle MW. Whittle's Gait Analysis-E-Book. Elsevier Health Sciences; 2012 Jul 13.
- ↑ 2.0 2.1 2.2 2.3 EWINS & COLLINS, 2014. Clinical Engineering. Sub- Chapter 25.1. Principles of kinematics and kinetics. Oxford Academic Press. Page 402-406.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Haentjens M. Instrumented Gait Analysis Course. Phsyioplus 2020
- ↑ Brunnekreef JJ, Van Uden CJ, van Moorsel S, Kooloos JG. Reliability of videotaped observational gait analysis in patients with orthopedic impairments. BMC musculoskeletal disorders. 2005 Dec;6(1):17.
- ↑ Cervical Motor Control Example . Available from: https://www.youtube.com/watch?v=HoLbn1NhUGk[last accessed 08/06/2020]
- ↑ Winter, D. A. Te Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological, 2nd edn (University of Waterloo Press, 1991).
- ↑ 7.0 7.1 7.2 7.3 Ancillao A. Modern functional evaluation methods for muscle strength and gait analysis. Cham, Switzerland: Springer International Publishing; 2018.
- ↑ Robertson GE, Caldwell GE, Hamill J, Kamen G, Whittlesey S. Research methods in biomechanics. Human kinetics; 2013 Nov 1.
- ↑ 9.0 9.1 AVEYARD, 2018. Doing a Literature Review in Health and Social Care: A Practical Guide. Fourth edition. Edition: Open University Press. UK. [online] [viewed 12/10/2019]. Available from: https://www.dawsonera.com/abstract/9780335248018
- ↑ FERNANDES et al., 2015. Test–retest reliability and minimal detectable change of three-dimensional gait analysis in chronic low back pain patients. Gait & Posture. N°42. Page 491-497. [online] [viewed 10/01/2020]. Available from: https://doi.org/10.1016/j.gaitpost.2015.08.002.
- ↑ 11.0 11.1 11.2 RICHARDS, 2018. The comprehensive textbook of clinical biomechanics. Second edition. ed. Amsterdam, The Netherlands: Elsevier.
- ↑ Lencioni T, Carpinella I, Rabuffetti M, Marzegan A, Ferrarin M. Human kinematic, kinetic and EMG data during different walking and stair ascending and descending tasks. Scientific data. 2019 Dec 6;6(1):1-0.
- ↑ SEMPLE et al., 2009. Tibialis posterior in health and disease: a review of structure and function with specific reference to electromyographic studies. Journal of Foot and Ankle Research. N°2. Page 24. [online] [viewed 10/01/2020].
- ↑ HERMENS et al., 2000. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. N°10. Page 361-374. [online] [viewed 12/10/2019].
- ↑ 15.0 15.1 Soderberg GL, Knutson LM. A guide for use and interpretation of kinesiologic electromyographic data. Physical therapy. 2000 May 1;80(5):485-98.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 Smith SL. Neuromuscular control in knee osteoarthritis (NEKO) (Doctoral dissertation, Glasgow Caledonian University).
- ↑ GRAHAM et al., 2012. Small sample research designs for evidence-based rehabilitation: issues and methods. Archives of Physical Medicine and Rehabilitation. N° 93. Page 111-116.
- ↑ KOLLMITZER et al., 1999. Reliability of surface electromyographic measurements. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. N°110. Page 725-734.