Deep Brain Stimulation: Difference between revisions
No edit summary |
(Added more references. Included more information to clarify some of the points raised.) |
||
| Line 6: | Line 6: | ||
== Introduction == | == Introduction == | ||
[[File:Deep brain stimulation.jpeg|right|frameless|354x354px]] | [[File:Deep brain stimulation.jpeg|right|frameless|354x354px]] | ||
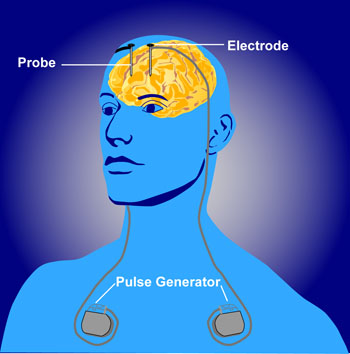
DBS involves the placement of electrodes into [[Basal Ganglia|deep brain structures]]. Current is administered through these electrodes by an implanted power pack, which can be remotely controlled by the subject and adjusted by physicians. In essence, DBS is like a [[Cardiac Implantable Electronic Devices (CIEDs)|pacemaker]] for the brain<ref name=":1">The Conversation Do brain interventions to treat disease change the essence of who we are? Available from: https://theconversation.com/do-brain-interventions-to-treat-disease-change-the-essence-of-who-we-are-45943 (accessed 19.5.2021)</ref>. | Deep brain stimulation (DBS) involves the placement of electrodes into [[Basal Ganglia|deep brain structures]]. Current is administered through these electrodes by an implanted power pack, which can be remotely controlled by the subject and adjusted by physicians. In essence, DBS is like a [[Cardiac Implantable Electronic Devices (CIEDs)|pacemaker]] for the brain<ref name=":1">The Conversation Do brain interventions to treat disease change the essence of who we are? Available from: https://theconversation.com/do-brain-interventions-to-treat-disease-change-the-essence-of-who-we-are-45943 (accessed 19.5.2021)</ref>. | ||
DBS is used in a variety of clinical settings, predominantly in patients with poorly controlled movement disorders. Although effective, its exact mode of function continues to be poorly understood<ref name=":0">Radiopedia Deep Brain Stimulation Available from: https://radiopaedia.org/articles/deep-brain-stimulation<nowiki/>(accessed 19.5.2021)</ref>. | |||
DBS is designed to alter the function of circuits in the [[Brain Anatomy|brain]]. It has been used with varying degrees of success in the treatment of [[Parkinson's|Parkinson’s disease]], dystonia, [[epilepsy]], obsessive-compulsive disorder and even [[depression]]. | DBS is designed to alter the function of circuits in the [[Brain Anatomy|brain]]. It has been used with varying degrees of success in the treatment of [[Parkinson's|Parkinson’s disease]], dystonia, [[epilepsy]], obsessive-compulsive disorder and even [[depression]].<ref>Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. ''Mov Disord''. (2006) 21(Suppl. 14):S284–9. doi: 10.1002/mds.20961</ref><ref name=":3">Walter BL, Vitek JL. Surgical treatment for Parkinson's disease. ''Lancet Neurol''. (2004) 3:719–28. doi: 10.1016/S1474-4422(04)00934-2</ref><ref name=":4">Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid A-L. Deep brain stimulation for Parkinson's disease: Surgical technique and perioperative management. ''Mov Disord''. (2006) 21:S247–58. doi: 10.1002/mds.20959</ref> | ||
Careful patient selection and target selection are essential if the procedure is to have good efficacy<ref name=":0" />. | Careful patient selection and target selection, accurate electrode placements and post-operative programming of DBE devices are essential if the procedure is to have good efficacy<ref name=":0" /><ref name=":3" /><ref name=":4" />. | ||
== Anatomy == | == Anatomy == | ||
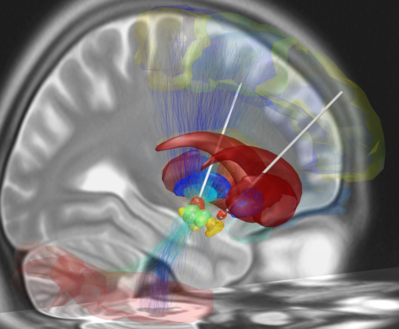
[[File:Deep brain stimulation electrode placement reconstruction.png|right|frameless|399x399px]] | [[File:Deep brain stimulation electrode placement reconstruction.png|right|frameless|399x399px|alt=|Image 2: Depicted is a reconstruction of bihemispheric DBS electrodes that have been surgically placed into the most common target structure for treatment of Parkinson's Disease, the subthalamic nucleus (orange).]] | ||
The DBS apparatus consists of electrodes implanted adjacent to specific deep brain structures, which are then connected to a pacemaker-like machine (pulse generator) that is implanted on the chest wall, via a subcutaneous wire. Stimulation parameters are then relayed by a computer to the pulse generator, appropriating proper amplitudes, frequencies, and pulse width. Common structures targeted by DBS include the subthalamic nucleus (STN), globus pallidus interna (GPi), and the ventral intermediate nucleus of the [[thalamus]] (VIM). | The DBS apparatus consists of electrodes implanted adjacent to specific deep brain structures, which are then connected to a pacemaker-like machine (pulse generator) that is implanted on the chest wall, via a subcutaneous wire<ref name=":2">Fariba K, Gupta V. [https://www.ncbi.nlm.nih.gov/books/NBK557847/ Deep Brain Stimulation.] StatPearls [Internet]. 2020 May 17.Available from: https://www.ncbi.nlm.nih.gov/books/NBK557847/ (accessed 19.5.2021)</ref>. Stimulation parameters are then relayed by a computer to the pulse generator, appropriating proper amplitudes, frequencies, and pulse width. Common structures targeted by DBS include the subthalamic nucleus (STN), globus pallidus interna (GPi), and the ventral intermediate nucleus of the [[thalamus]] (VIM).<ref>Shehab S, D'souza C, Ljubisavljevic M, Redgrave P. High-frequency electrical stimulation of the subthalamic nucleus excites target structures in a model using c-fos immunohistochemistry. Neuroscience. 2014 Jun 13;270:212-25. doi: 10.1016/j.neuroscience.2014.04.016. Epub 2014 Apr 19. PMID: 24755486.</ref><ref name=":5">Lavian H, Ben-Porat H, Korngreen A. High and low frequency stimulation of the subthalamic nucleus induce prolonged changes in subthalamic and globus pallidus neurons. Frontiers in systems neuroscience. 2013 Dec 18;7:73.</ref> | ||
The precise mechanism of the resultant therapeutic effects of deep brain stimulation remains unclear. Imaging and physiologic studies | The precise mechanism of the resultant therapeutic effects of deep brain stimulation remains unclear, although some studies suggested that high frequency stimulation (HFS) in the subthalamic nucleus reduces the firing rate of STN neurons and is responsible for the suppression of symptoms seen in the neurologic conditions such as Parkinson's Disease. <ref>Filali, M., Hutchison, W. D., Palter, V. N., Lozano, A. M., and Dostrovsky, J. O. (2004). Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. ''Exp. Brain Res.'' 156, 274–281. doi: 10.1007/s00221-003-1784-y</ref> However, some DBS Imaging and physiologic studies sugest the hypothesis that the ultimate effect of deep brain stimulation is the increase of the firing of the targeted [[Neurone|neuron]]<nowiki/>s.<ref>Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229-57.</ref> | ||
Depicted on the image on the right is a reconstruction of bihemispheric DBS electrodes that have been surgically placed into the most common target structure for treatment of Parkinson's Disease, the subthalamic nucleus (orange). Other subcortical structures include the red nucleus (green), the substantia nigra (yellow), the internal (cyan) and external (blue) pallidum and the striatum (red). | |||
== Indications == | == Indications == | ||
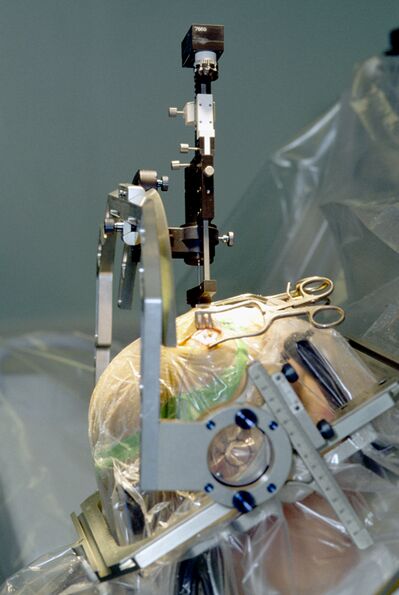
[[File:Parkinson surgery.jpeg|right|frameless|595x595px]] | [[File:Parkinson surgery.jpeg|right|frameless|595x595px|alt=|Image 3: Stereotactic procedure for inserting an electrode for deep brain stimulation in a patient with Parkinson's disease.]] | ||
1.[[Parkinson's|Parkinson disease]] | 1.[[Parkinson's|Parkinson disease]]<ref name=":5" /> | ||
* subthalamic nucleus | * subthalamic nucleus | ||
| Line 33: | Line 33: | ||
** ventral intermediate nucleus | ** ventral intermediate nucleus | ||
2. Dystonia (cervical dystonia and tardive dystonia) | 2. Dystonia (cervical dystonia and tardive dystonia)<ref>Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K. Deep brain stimulation programming for movement disorders: current concepts and evidence-based strategies. Frontiers in neurology. 2019 May 21;10:410.</ref> | ||
* globus pallidus (pars interna) | * globus pallidus (pars interna) | ||
| Line 40: | Line 40: | ||
** hypothalamus | ** hypothalamus | ||
3. [[Chronic Pain and the Brain|Chronic pain]] | 3. [[Chronic Pain and the Brain|Chronic pain]]<ref>Boccard SG, Pereira EA, Aziz TZ. Deep brain stimulation for chronic pain. Journal of Clinical Neuroscience. 2015 Oct 1;22(10):1537-43.</ref> | ||
* thalamus | * thalamus | ||
* periaqueductal grey matter | * periaqueductal grey matter | ||
4. Neuropsychiatric disorders: largely experimental<ref name=":0" /> | 4. Neuropsychiatric disorders: largely experimental<ref name=":0" /> | ||
== Significance == | == Significance == | ||
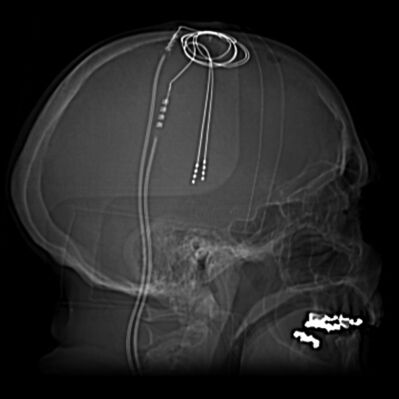
[[File:Deep-brain-stimulation.jpeg|right|frameless|399x399px]] | [[File:Deep-brain-stimulation.jpeg|right|frameless|399x399px|alt=|Image 4: Scout and CT images demonstrate bilateral deep brain stimulation electrodes extending to the region of the subthalamic nuclei. NB Scout is an preliminary film taken of a body region before a definitive imaging study–eg, an SF of the chest before a CT; 'scouts' serve to establish a baseline and are used before angiography, CT, MRI]] | ||
DBS has the potential for many applications, and has specifically been heralded as offering "a new life for people with Parkinson disease." | DBS has the potential for many applications, and has specifically been heralded as offering "a new life for people with Parkinson disease."<ref>Chou KL, Grube S, Patil PG, Patil P. Deep brain stimulation: A new life for people with Parkinson's, dystonia, and essential Tremor. Demos Medical Publishing; 2011 Dec 21.</ref> | ||
* DBS has already been awarded FDA approval for essential tremor, dystonia, Parkinson disease, and treatment-refractory obsessive-compulsive disease. Ongoing studies are investigating the possible off-label use in addiction, autism, anorexia nervosa, anxiety disorders, and schizophrenia<ref name=":2" />. | * DBS has already been awarded FDA approval for essential tremor, dystonia, Parkinson disease, and treatment-refractory obsessive-compulsive disease. Ongoing studies are investigating the possible off-label use in addiction, autism, anorexia nervosa, anxiety disorders, and schizophrenia<ref name=":2" />. | ||
* DBS has been a life-restoring therapeutic technique for thousands of patients with Parkinson’s disease with severely impaired motor and cognitive function, and it promises to dramatically improve the lives of people suffering from some other psychological and neurological disorders, including obsessive compulsive disorder, treatment-resistant depression, and Tourette’s syndrome. | * DBS has been a life-restoring therapeutic technique for thousands of patients with Parkinson’s disease with severely impaired motor and cognitive function,<ref>Groiss SJ, Wojtecki L, Südmeyer M, Schnitzler A. Deep brain stimulation in Parkinson’s disease. Therapeutic advances in neurological disorders. 2009 Nov;2(6):379-91.</ref> and it promises to dramatically improve the lives of people suffering from some other psychological and neurological disorders, including obsessive compulsive disorder, treatment-resistant depression, and Tourette’s syndrome.<ref>Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, Malaty IA, Goodman WK, Gilbert DM, Walker HC, Mink JW. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA neurology. 2013 Jan 1;70(1):85-94.</ref> | ||
* DBS can in seconds restore normal function to a person virtually paralyzed by a dopamine deficiency, contorted by uncontrollable tremor and muscular contraction. The transformation is miraculous. However, stimulation may result in unanticipated side-effects, altering mood, preferences, desires, emotion or cognition. .<ref name=":1" /> | * DBS can in seconds restore normal function to a person virtually paralyzed by a dopamine deficiency, contorted by uncontrollable tremor and muscular contraction. The transformation is miraculous. However, stimulation may result in unanticipated side-effects, altering mood, preferences, desires, emotion or cognition. .<ref name=":1" /> | ||
With neurodegenerative movement disorders like Parkinson’s disease, the ability to deliver the right dose of stimulation where it is needed can make a remarkable difference in controlling an individual patient’s symptoms. | With neurodegenerative movement disorders like Parkinson’s disease, the ability to deliver the right dose of stimulation where it is needed can make a remarkable difference in controlling an individual patient’s symptoms. | ||
| Line 63: | Line 60: | ||
The latest DBSs (an upgrade to earlier systems available for years in both the U.S. and Europe) is approved as an add-on treatment for levodopa-responsive Parkinson’s patients, for whom levodopa alone is insufficient. It is approved for stimulation of both sides of the subthalamic nucleus and the internal globus pallidus, which are brain regions that play key roles in motor function. | The latest DBSs (an upgrade to earlier systems available for years in both the U.S. and Europe) is approved as an add-on treatment for levodopa-responsive Parkinson’s patients, for whom levodopa alone is insufficient. It is approved for stimulation of both sides of the subthalamic nucleus and the internal globus pallidus, which are brain regions that play key roles in motor function. | ||
== | == New DBS system == | ||
The new DBS System’s pulse generator stimulates the brain with a pre-determined program of mild electrical impulses. | The new DBS System’s pulse generator stimulates the brain with a pre-determined program of mild electrical impulses. | ||
Revision as of 15:18, 16 November 2021
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton, Joseph Ayotunde Aderonmu and Pacifique Dusabeyezu
Introduction[edit | edit source]
Deep brain stimulation (DBS) involves the placement of electrodes into deep brain structures. Current is administered through these electrodes by an implanted power pack, which can be remotely controlled by the subject and adjusted by physicians. In essence, DBS is like a pacemaker for the brain[1].
DBS is used in a variety of clinical settings, predominantly in patients with poorly controlled movement disorders. Although effective, its exact mode of function continues to be poorly understood[2].
DBS is designed to alter the function of circuits in the brain. It has been used with varying degrees of success in the treatment of Parkinson’s disease, dystonia, epilepsy, obsessive-compulsive disorder and even depression.[3][4][5]
Careful patient selection and target selection, accurate electrode placements and post-operative programming of DBE devices are essential if the procedure is to have good efficacy[2][4][5].
Anatomy[edit | edit source]
The DBS apparatus consists of electrodes implanted adjacent to specific deep brain structures, which are then connected to a pacemaker-like machine (pulse generator) that is implanted on the chest wall, via a subcutaneous wire[6]. Stimulation parameters are then relayed by a computer to the pulse generator, appropriating proper amplitudes, frequencies, and pulse width. Common structures targeted by DBS include the subthalamic nucleus (STN), globus pallidus interna (GPi), and the ventral intermediate nucleus of the thalamus (VIM).[7][8]
The precise mechanism of the resultant therapeutic effects of deep brain stimulation remains unclear, although some studies suggested that high frequency stimulation (HFS) in the subthalamic nucleus reduces the firing rate of STN neurons and is responsible for the suppression of symptoms seen in the neurologic conditions such as Parkinson's Disease. [9] However, some DBS Imaging and physiologic studies sugest the hypothesis that the ultimate effect of deep brain stimulation is the increase of the firing of the targeted neurons.[10]
Depicted on the image on the right is a reconstruction of bihemispheric DBS electrodes that have been surgically placed into the most common target structure for treatment of Parkinson's Disease, the subthalamic nucleus (orange). Other subcortical structures include the red nucleus (green), the substantia nigra (yellow), the internal (cyan) and external (blue) pallidum and the striatum (red).
Indications[edit | edit source]
- subthalamic nucleus
- pedunculopontine nucleus
- globus pallidus (pars interna)
- Severe essential tremor
- ventral intermediate nucleus
2. Dystonia (cervical dystonia and tardive dystonia)[11]
- globus pallidus (pars interna)
- Cluster headaches
- hypothalamus
- thalamus
- periaqueductal grey matter
4. Neuropsychiatric disorders: largely experimental[2]
Significance[edit | edit source]
DBS has the potential for many applications, and has specifically been heralded as offering "a new life for people with Parkinson disease."[13]
- DBS has already been awarded FDA approval for essential tremor, dystonia, Parkinson disease, and treatment-refractory obsessive-compulsive disease. Ongoing studies are investigating the possible off-label use in addiction, autism, anorexia nervosa, anxiety disorders, and schizophrenia[6].
- DBS has been a life-restoring therapeutic technique for thousands of patients with Parkinson’s disease with severely impaired motor and cognitive function,[14] and it promises to dramatically improve the lives of people suffering from some other psychological and neurological disorders, including obsessive compulsive disorder, treatment-resistant depression, and Tourette’s syndrome.[15]
- DBS can in seconds restore normal function to a person virtually paralyzed by a dopamine deficiency, contorted by uncontrollable tremor and muscular contraction. The transformation is miraculous. However, stimulation may result in unanticipated side-effects, altering mood, preferences, desires, emotion or cognition. .[1]
With neurodegenerative movement disorders like Parkinson’s disease, the ability to deliver the right dose of stimulation where it is needed can make a remarkable difference in controlling an individual patient’s symptoms.
The latest DBSs (an upgrade to earlier systems available for years in both the U.S. and Europe) is approved as an add-on treatment for levodopa-responsive Parkinson’s patients, for whom levodopa alone is insufficient. It is approved for stimulation of both sides of the subthalamic nucleus and the internal globus pallidus, which are brain regions that play key roles in motor function.
New DBS system[edit | edit source]
The new DBS System’s pulse generator stimulates the brain with a pre-determined program of mild electrical impulses.
- The new device is based on cochlear implant technology, which precisely stimulates auditory nerves to replicate hearing. Earlier models, in contrast, rely upon pacemaker technology.
- Using Bluetooth wireless technology, individual DBS stimulation programs can be fine-tuned and adjusted as needed, to address changes in symptoms that occur throughout Parkinson’s progression by targeting different brain regions. In this way, physicians and patients can work together to better optimize therapy for each individual[16].
It is estimated that 208,000 deep brain stimulation (DBS) devices have been implanted to address neurological and neuropsychiatric disorders worldwide[17]
References[edit | edit source]
- ↑ 1.0 1.1 The Conversation Do brain interventions to treat disease change the essence of who we are? Available from: https://theconversation.com/do-brain-interventions-to-treat-disease-change-the-essence-of-who-we-are-45943 (accessed 19.5.2021)
- ↑ 2.0 2.1 2.2 Radiopedia Deep Brain Stimulation Available from: https://radiopaedia.org/articles/deep-brain-stimulation(accessed 19.5.2021)
- ↑ Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. Mov Disord. (2006) 21(Suppl. 14):S284–9. doi: 10.1002/mds.20961
- ↑ 4.0 4.1 Walter BL, Vitek JL. Surgical treatment for Parkinson's disease. Lancet Neurol. (2004) 3:719–28. doi: 10.1016/S1474-4422(04)00934-2
- ↑ 5.0 5.1 Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid A-L. Deep brain stimulation for Parkinson's disease: Surgical technique and perioperative management. Mov Disord. (2006) 21:S247–58. doi: 10.1002/mds.20959
- ↑ 6.0 6.1 Fariba K, Gupta V. Deep Brain Stimulation. StatPearls [Internet]. 2020 May 17.Available from: https://www.ncbi.nlm.nih.gov/books/NBK557847/ (accessed 19.5.2021)
- ↑ Shehab S, D'souza C, Ljubisavljevic M, Redgrave P. High-frequency electrical stimulation of the subthalamic nucleus excites target structures in a model using c-fos immunohistochemistry. Neuroscience. 2014 Jun 13;270:212-25. doi: 10.1016/j.neuroscience.2014.04.016. Epub 2014 Apr 19. PMID: 24755486.
- ↑ 8.0 8.1 Lavian H, Ben-Porat H, Korngreen A. High and low frequency stimulation of the subthalamic nucleus induce prolonged changes in subthalamic and globus pallidus neurons. Frontiers in systems neuroscience. 2013 Dec 18;7:73.
- ↑ Filali, M., Hutchison, W. D., Palter, V. N., Lozano, A. M., and Dostrovsky, J. O. (2004). Stimulation-induced inhibition of neuronal firing in human subthalamic nucleus. Exp. Brain Res. 156, 274–281. doi: 10.1007/s00221-003-1784-y
- ↑ Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229-57.
- ↑ Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K. Deep brain stimulation programming for movement disorders: current concepts and evidence-based strategies. Frontiers in neurology. 2019 May 21;10:410.
- ↑ Boccard SG, Pereira EA, Aziz TZ. Deep brain stimulation for chronic pain. Journal of Clinical Neuroscience. 2015 Oct 1;22(10):1537-43.
- ↑ Chou KL, Grube S, Patil PG, Patil P. Deep brain stimulation: A new life for people with Parkinson's, dystonia, and essential Tremor. Demos Medical Publishing; 2011 Dec 21.
- ↑ Groiss SJ, Wojtecki L, Südmeyer M, Schnitzler A. Deep brain stimulation in Parkinson’s disease. Therapeutic advances in neurological disorders. 2009 Nov;2(6):379-91.
- ↑ Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, Malaty IA, Goodman WK, Gilbert DM, Walker HC, Mink JW. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA neurology. 2013 Jan 1;70(1):85-94.
- ↑ PD news today First US Patient Receives New Vercise Genus Deep Brain Stimulation Device Available from:https://parkinsonsnewstoday.com/2021/02/23/first-vercise-genus-deep-brain-stimulation-device-parkinsons-disease-implanted-us-patient/ (accessed 19.5.2021)
- ↑ Vedam-Mai V, Deisseroth K, Giordano J, Lazaro-Munoz G, Chiong W, Suthana N, Langevin JP, Gill J, Goodman W, Provenza NR, Halpern CH. Proceedings of the Eighth Annual Deep Brain Stimulation Think Tank: Advances in Optogenetics, Ethical Issues Affecting DBS Research, Neuromodulatory Approaches for Depression, Adaptive Neurostimulation, and Emerging DBS Technologies. Frontiers in Human Neuroscience. 2021 Apr 19;15:169.Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2021.644593/full(accessed 19.5.2021)