Spasticity Management in Spinal Cord Injury
Original Editor - Ewa Jaraczewska based on the course by Melanie Harding
Top Contributors - Ewa Jaraczewska, Jess Bell and Kim Jackson
Introduction[edit | edit source]
Spasticity is a common symptom in people with a spinal cord injury (SCI).[1]It can often be underdiagnosed. According to Holtz et al.[2] 35% of patients with problematic spasticity require some kind of management within one year from the initial spinal cord injury. [2]Problematic spasticity can be painful and interfere with a person's mobility and function, including body hygiene or toileting. It is most common in patients diagnosed with ASIA grades B through D at the cervical level. Other patients can benefit from spasticity to facilitate their trunk stability for postural control, basic and complex activities such as standing, transfers, and activities of daily living. [3] This article discusses definitions, assessment methods and management of spasticity in a spinal cord injury.
Definitions of Spasticity[edit | edit source]
According to the European consensus on the concepts and measurement of the pathophysiological neuromuscular responses to passive muscle stretch, the term spasticity or hypertonia due to an impaired neuromuscular response during passive stretch should be replaced by the term hyper-resistance. It further defines hyperresistance as a consequence of the following:
- non-neural changes occurring in the tissue, including elasticity, viscosity, muscle shortening, and contracture.
- neural changes in the central nervous system (CNS) characterising in "velocity-dependent stretch hyperreflexia and non-velocity dependent involuntary background activation".[4]
The following are current definitions of spasticity:
“A motor disorder characterised by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neuron syndrome” JW Lance, 1980 [5]
"Disordered sensorimotor control, resulting from an upper motor neurone lesion, presenting as intermittent or sustained involuntary activation of muscles." 2005 SPASM (Support Programme for Assembly of a database for Spasticity Measurement) consortium [6]
“Spasticity refers to velocity-dependent stretch hyperreflexia as part of hyper-resistance.” 2016 European consensus [4]
Clinicians must be aware that the term spasticity can only be used when it is clearly defined by the type of changes that have occurred. The term stiffness is reserved for the tissue-related contributions to hyper-resistance. [4]
Neurological Components of Spasticity[edit | edit source]
- An upper motor neuron lesion to the spinal cord [7]
- Disinhibition occurring at the spinal reflex loop as a direct result of the neurological insult [4]
Spasticity vs Muscular Spasms[edit | edit source]
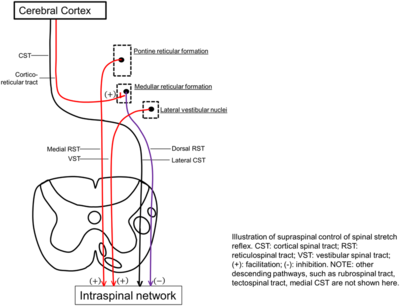
Spasticity refers to a loss of supraspinal inhibitory influences on muscle stretch reflexes provided by corticospinal, corticoreticular, and dorsal reticulospinal tracts.
Muscular spasms are muscular contractions that are involuntary, uncontrolled, periodic, and non-sustained.
Epidemiology of Spasticity[edit | edit source]
- It occurs more in patients with tetraplegia. [7]
- 48% of patients with SCI present with spasticity upon admission to the acute inpatient rehabilitation unit. [8]
- 46-65% of persons with traumatic SCI reported spasticity at discharge. [3][8]
- Problematic spasticity has been reported by 79% of patients with cervical injuries, 69% of patients with thoracic SCI and 22% of patients with lumbosacral SCI.[3]
- Spasticity is less common in lesions between T12-L2. [3]The spasticity develops in:
- 87% of patients with a cervical level of injury
- 85% of patients with thoracic injury
- 57% of patients with lumbar injury
- No spasticity develops below L2. [7]
- Medications are commonly used to manage spasticity in a spinal cord injury. [8]
Read about facts and figures on spinal cord injury here.
Phases in Spasticity Development[edit | edit source]
- Initial phase of areflexia.[9]
- Spinal shock: acute SCI with flaccid tone below the level of injury.[10]
- Return of reflexes. [9]
- Hyperreflexia, spasms, and clonus are emerging. [9]
Spasticity Assessment[edit | edit source]
Considerations prior to the assessment: [7]
- Spasticity may vary throughout the 24-hour day, the days of the week and even during a treatment session.
- It can present in all 4 limbs and the trunk and may be in the flexors, extensors, rotators, adductors or abductors. All assessments should be performed with the muscle at rest(relaxed) for accurate results.
- Spasticity can increase or decrease due to specific movements, activities, stressors or positions.
- Assess the effect of spasticity on function and ADL, for example, gait, bed mobility, transfers, sleep, bladder and bowel function.
Spasticity assessment goals:[7]
- To understand the patient and the caregiver's perception of spasticity
- To identify where the problem may be
- To validate how realistic the desired outcome
- To evaluate treatment interventions[11]
Outcome Measures[edit | edit source]
There are many methods developed for spasticity measurement. However, the spasticity evaluation process remains problematic. [12]Spasticity measurement methods can be classified as following:[12]
1. Clinical scales
- The Modified Ashworth Scale (MAS) [13]
- Six-point ordinal scale for grading resistance encountered during passive movement stretching at increasing velocity
- 0=no increase in muscle tone
- 1=slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion (ROM) when the affected part(s) is moved in flexion or extension.
- 1+= slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM.
- 2=more marked increase in muscle tone through most ROM, but the affected part(s) is easily moved.
- 3= considerable increase in muscle tone; passive movement is difficult.
- 4=affected part(s) rigid in flexion or extension.
- Adequate reliability in assessing lower-extremity spasticity in patients with SCI
- You can access MAS here.
- Six-point ordinal scale for grading resistance encountered during passive movement stretching at increasing velocity
- The Modified Tardieu Scale (MTS) [14]
- Validated in SCI
- Assess three key components: the velocity of stretch, the quality of the muscle reaction, and the angle of the muscle reaction
- Consider being superior to the MAS.
- Can distinguish between neural and peripheral contributions to spasticity.
- "Quantifies muscle tone at specified velocities."
- Three speeds of passive movements are used for the joint angle measurements: very slow, a passive fall of the limb under gravity's influence, and as fast as possible.
- A complementary tool for planning the treatment for people with SCI.
- Here is the link to the instrument
- The Penn Spasm Frequency Scale (PSFS)
- A self-reported measure of the frequency of reported muscle spasms which is commonly used to quantify spasticity
- Contains two parts: spasm frequency and spasm severity
- Spasm frequency:
- 0= No spasms
- 1= One or a few spasms per day
- 2=between 1 and 5 spasms per day
- 3= 5 or <10 spasms per day
- 4= ten or more spasms per day or continuous contraction
- Spasm severity: mild (1), moderate (2), and severe (3)
- Poorly correlated with MAS
- More information about PSFS can be found here.
- Spastic Paraplegia Rating Scale (SPRS)
- It takes 15 minutes to administer and requires no special equipment.[15]
- A high interrater correlation
- 13 variables to measure the severity of spastic paraplegia. [16]
- Rates functional impairment by assessing walking ability, muscle power, spasticity, pain and urinary function[17]
- Score for each variable ranges from 0(normal) to 4(severe impairment). [16]
- The maximum total score is 52.
2. Biomechanical methods
- Pendulum Test
- A simple method to quantify the severity of spasticity.
- The ratio between the initial flexion and the final resting position of the knee joint measured by goniometers can be used to quantify the severity of spasticity. [18]
- The results depend on the person's seating position and ability to relax fully.[18]
- The test can only evaluate spasticity in the knee muscles.
- Clinician is not able to delineate the viscoelastic changes vs. the velocity-dependent resistance due to spasticity
3. Neurophysiological-Electrophysiological methods
- Spinal Cord Assessment Tool for Spastic Reflexes (SCATS)
- Used to assess clonus, flexor spasms, and extensor spasms.
- Three degrees of spasm rated between zero to three: no spasm (0) to severe spasm (3)
- Reliable clinical measurement tool for assessing spasm activity and spastic hypertonia in patients with SCI [19]
- Detailed information and link to the instrument is here.
Characteristic of Spasticity in SCI[edit | edit source]
Spasticity is found to negatively influence a person's quality of life by limiting their abilities to perform activities of daily living, inhibiting self-care, causing pain and affecting patients' safety. There may be beneficial effects of spasticity on improving trunk stability in sitting and standing, facilitating transfers and walking, and increasing venous return, thus reducing the incidence of deep vein thrombosis. The presence and severity of spasticity may vary throughout the day, and well-known factors can aggravate or alleviate the presence and symptoms of spasticity.
Aggravating Factors[edit | edit source]
The following are the factors negatively affecting spasticity:
- Immobility or remaining too long in one position
- Pain (a fracture, muscle overuse injury, pressure sore, kidney stone, bladder infection, appendicitis, labour)
- Overfull bladder or bowel
- Constipation
- Various infections: UTI, ingrown toenail, and decubitus ulcer
- Anxiety, stress-emotional disorders
- Sudden heat and moisture changes in the environment, cold weather, oncoming rain
- Pressure on the ball of the foot (stimulates the plantar reflex)
- Bed or wheelchair positioning with not enough hip and knee flexion
- Heterotrophic ossification (HO)
- Contractures
- Syringomyelia
- Deep-vein thrombosis
- Fever
- Tight-fitting clothes or urinary leg bag straps
- Uncomfortable orthotics
Alleviating Factors[edit | edit source]
- Slow passive movements and stretches when performed regularly [7][20]
- Standing and weight bearing [21]
- Trunk rhythmical side flexion and extension combined with trunk torsion as during horse riding [22]
- The patient’s physical activity and treatment, including stretching and bending[23]
- Proper positioning in bed and chair
- Pain management
- Bladder and bowel management
- Stress management
- Deep breathing and relaxation
- Cold/Ice[20]
General Principles in Spasticity Management[edit | edit source]
Spasticity should be addressed when it causes detriment to the patient, such as pain, functional limitations, formation of muscle contracture, or positional limitations.[3]
Early interventions for the prevention and treatment of post-spinal cord injury spasticity are recommended. [9]
When planning the treatment, clinicians should consider the following factors related to spasticity:[3]
- The quality of the spasticity
- Severity of the spasticity
- Distribution of spasticity
- Alleviating factors
- Aggravating factors
- Patients’ perception/explanations of their spasticity
Therapeutic Management of Spasticity[edit | edit source]
Self-Management[edit | edit source]
The patient and the caregiver must understand the patient's medical conditions and therapeutic interventions to select effective treatment and achieve the best outcome. [24] The patient should be instructed on self-management techniques for spasticity control. The instructions should include the following:
- Learning how to recognise and prevent factors that may aggravate spasticity and spasms [24]
- Performance of regular stretching, including prone position to maintain muscle length
- Pushing down on the knee to flatten the foot on the floor or foot plate to stop clonus
- Using the spasticity for function
Weight-Bearing[edit | edit source]
The long-term effectiveness of weight-bearing activities has not been well-researched and is frequently questioned.[25] The study by Bohannon[26]indicated an immediate reduction in lower extremity spasticity and spasms reduction lasting a few hours.
The following is an example of weight-bearing activities and its outcome:
Tilt table or standing frame:
- Benefits exceed the stretching alone
- The underlying mechanism includes the influence of cutaneous and joint receptor input to the spinal motor neurons, which results in decreased excitability
- A prolonged stretch of ankle plantar flexor muscles causes spasticity reduction in the lower extremities
Positioning[edit | edit source]
Positioning in bed or in a wheelchair is vital to maintain muscle length. There are clinical reports of the effectiveness of positioning on spasticity reduction, but scientific proof is lacking. [25] There are factors leading to an increase in spasticity when sitting in the wheelchair:
- Poor trunk stability and the effort required to compensate for it.
- Pain as a result of poor positioning.
- Muscle shortening due to poor positioning becomes a trigger point if stretched.
To decrease abnormal tone influences, the following should be considered:
- Standard solutions for wheelchair positioning are usually not applicable. [27]
- A team approach between occupational therapists and physiotherapists is essential.
- A good knowledge of the patient's physical impairment, wheelchair adaptation, seating systems and cushions is a must.
- Understanding the individual's demands and wishes will influence the team's decision.
- A golden rule is to change the position throughout the day.[24]
Upper Limb Spasticity Management[edit | edit source]
Learn more about upper limb spasticity management from here. This Physiopedia article discusses using modalities in upper limb management in tetraplegia.
Medication for Pain, Spasticity and Infection Depending on Cause[edit | edit source]
Baclofen tablets:[23]
- Only a small portion of the active substance penetrates the blood-brain barrier, hence the limited effect
- Strong side effects, including sedation, nausea, dizziness and difficulty in breathing
- Sudden interruption in taking the medication may cause epileptic seizures, psychosis and hyperthermia
Tizanidine:
- Offers a pronounced muscle relaxant effect
- Suppresses polysynaptic reflexes in a complete SCI
- Can be combined with baclofen[28]
- Same side effects as baclofen
Cannabis products:
- Conflicting results [23]
- According to Karst et al., cannabis "is not recommended for treating spasticity because of the narrow therapeutic range and risk of side effects and dependence" [29]
Botulinum toxin injections:
- Used when spasticity is present in a few muscles or a limited muscle group
- Temporary blocks the connection between the nerve terminal and muscle fibre by stopping the presynaptic release of acetylcholine from nerve terminals
- Through assessment with a detailed analysis and description of the function is essential for a good outcome when the toxin is injected into a particular muscle.
- The medication is delivered into the subarachnoid space by a programmable pump via a catheter system
- The pump must be refilled, and the dose of the medicine can be adjusted
Surgical Management[edit | edit source]
- Z plasty or tenotomies to lengthen the muscle: [30][31]
- The most common locations for tendon surgery in the lower extremity include hip flexion, hip adduction, knee flexion contracture, knee extension contracture, the equinovarus deformity at the ankle, toes flexion contracture, and hallux extension.
- In the upper extremity, the release procedure may include flexor digitorum profundus and superficialis (FDP and FDS), flexor carpi radialis (FCR), and flexor pollicis longus (FPL), and Z-lengthening of flexor carpi ulnaris (FCU)
Positive Effects of Spasticity on Function[edit | edit source]
Gait training:
- Extensor spasticity can assist with gait and sit-to-stand and transfers. However, severe spasticity increases energy usage, limits walking speed and affects safety. These factors may outweigh the advantages of walking over the speed and ease of a wheelchair. [7]
- More on gait after spinal cord injury can be found here.
Bed mobility:
- Trunk spasticity can facilitate rolling over or sitting up[7]
Transfers:
- Spasticity can assist with lifting legs up onto the bed [7]
Coughing:
- Spasticity can be used to stimulate a more effective cough[7]
Hand Grip:
- Spasticity in finger flexors can be stimulated to assist in strengthening the tenodesis grip [7]
Resources[edit | edit source]
- Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord. 2006 Dec;44(12):708-22.
- Balci BP. Spasticity Measurement. Noro Psikiyatr Ars. 2018;55(Suppl 1):S49-S53.
- Postural Assessment And Seating Systems for People with Spinal Cord Injury.
- Positioning and General Management of Upper Limbs in Spinal Cord Injury
- SPINAL CORD INJURY GUIDELINES 2019
References[edit | edit source]
- ↑ Baunsgaard CB, Nissen UV, Christensen KB, Biering-Sørensen F. Modified Ashworth scale and spasm frequency score in spinal cord injury: reliability and correlation. Spinal Cord. 2016 Sep;54(9):702-8.
- ↑ 2.0 2.1 Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and Effect of Problematic Spasticity After Traumatic Spinal Cord Injury. Arch Phys Med Rehabil. 2017 Jun;98(6):1132-1138.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Billington ZJ, Henke AM, Gater DR Jr. Spasticity Management after Spinal Cord Injury: The Here and Now. J Pers Med. 2022 May 17;12(5):808.
- ↑ 4.0 4.1 4.2 4.3 Noort JCVD, Bar-On L, Aertbeliën E, Bonikowski M, Braendvik SM, Broström EW, Buizer AI, Burridge JH, Campenhout AV, Dan B, Fleuren JF, Grunt S, Heinen F, Horemans HL, Jansen C, Kranzl A, Krautwurst BK, Krogt MVD, Lara SL, Lidbeck CM, Lin J-P, Martinez I, Meskers C, Metaxiotis D, Molenaers G, Patikas DA, Rémy-Néris O, Roeleveld K, Shortland AP, Sikkens J, Sloot L, Vermeulen RJ, Wimmer C, Schröder AS, Schless S, Becher JG, Desloovere K, Harlaar J. European consensus on the concepts and measurement of the pathophysiological neuromuscular responses to passive muscle stretch. Eur J Neurol. 2017.
- ↑ Lance JW. Symposium synopsis. In Feldman RG, Young RR, Koella WP, editors. Spasticity: disordered motor control. Yearbook Medical, Chicago; 1980, pp. 485–494.
- ↑ Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, Hermens H, Johnson GR. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005 Jan 7-21;27(1-2):2-6.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 Harding M. General Principles of Spasticity Management in Spinal Cord Injury. Plus 2023
- ↑ 8.0 8.1 8.2 Dragojlovic N, Romanoski NL, Verduzco-Gutierrez M, Francisco GE. Prevalence and Treatment Characteristics of Spastic Hypertonia on First-Time Admission to Acute Inpatient Rehabilitation. Am J Phys Med Rehabil. 2022 Apr 1;101(4):348-352.
- ↑ 9.0 9.1 9.2 9.3 Stampas A, Hook M, Korupolu R, Jethani L, Kaner MT, Pemberton E, Li S, Francisco GE. Evidence of treating spasticity before it develops: a systematic review of spasticity outcomes in acute spinal cord injury interventional trials. Ther Adv Neurol Disord. 2022 Feb 17;15:17562864211070657.
- ↑ Ko HY. Revisit Spinal Shock: Pattern of Reflex Evolution during Spinal Shock. Korean J Neurotrauma. 2018 Oct;14(2):47-54.
- ↑ Rahimi F, Eyvazpour R, Salahshour N, Azghani MR. Objective assessment of spasticity by pendulum test: a systematic review on methods of implementation and outcome measures. Biomed Eng Online. 2020 Nov 9;19(1):82.
- ↑ 12.0 12.1 Balci BP. Spasticity Measurement. Noro Psikiyatr Ars. 2018;55(Suppl 1):S49-S53.
- ↑ Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987 Feb;67(2):206-7.
- ↑ Akpinar P, Atici A, Ozkan FU, Aktas I, Kulcu DG, Sarı A, Durmus B. Reliability of the Modified Ashworth Scale and Modified Tardieu Scale in patients with spinal cord injuries. Spinal Cord. 2017 Oct;55(10):944-949.
- ↑ Schüle R, Holland-Letz T, Klimpe S, Kassubek J, Klopstock T, Mall V, Otto S, Winner B, Schöls L. The Spastic Paraplegia Rating Scale (SPRS): a reliable and valid measure of disease severity. Neurology. 2006 Aug 8;67(3):430-4.
- ↑ 16.0 16.1 Galvão CRC, Cavalcante PMA, Olinda R, Graciani Z, Zatz M, Kok F, Santos S, Lancman S. Motor impairment in a rare form of spastic paraplegia (Spoan syndrome): a 10-year follow-up. BMC Neurol. 2019 Oct 27;19(1):256.
- ↑ Chou CT, Soong BW, Lin KP, Tsai YS, Jih KY, Liao YC, Lee YC. Clinical characteristics of Taiwanese patients with Hereditary spastic paraplegia type 5. Ann Clin Transl Neurol. 2020 Apr;7(4):486-496.
- ↑ 18.0 18.1 Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: a review. Spinal Cord. 2006 Dec;44(12):708-22.
- ↑ Akpinar P, Atici A, Ozkan FU, Aktas I, Kulcu DG, Kurt KN. Reliability of the Spinal Cord Assessment Tool for Spastic Reflexes. Arch Phys Med Rehabil. 2017 Jun;98(6):1113-1118.
- ↑ 20.0 20.1 Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal cord. 2005 Oct;43(10):577-86.
- ↑ Adams MM, Hicks AL. Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. The journal of spinal cord medicine. 2011 Sep 1;34(5):488-94.
- ↑ Lechner HE, Feldhaus S, Gudmundsen L, Hegemann D, Michel D, Zäch GA, Knecht H. The short-term effect of hippotherapy on spasticity in patients with spinal cord injury. Spinal cord. 2003 Sep;41(9):502-5.
- ↑ 23.0 23.1 23.2 Rekand T, Hagen EM, Grønning M. Spasticity following spinal cord injury. Tidsskrift for Den norske legeforening. 2012 Apr 30.
- ↑ 24.0 24.1 24.2 Mansoor Rayegani S, Babaee M, Ahmad Raeissadat S. Rehabilitation Medicine Management of Spasticity [Internet]. Neurostimulation and Neuromodulation in Contemporary Therapeutic Practice. IntechOpen; 2020. Available from https://www.intechopen.com/chapters/72741 [last access 24.07.2023]
- ↑ 25.0 25.1 Jozefczyk PB. The management of focal spasticity. Clin Neuropharmacol. 2002 May-Jun;25(3):158-73.
- ↑ Bohannon RW. Tilt table standing for reducing spasticity after spinal cord injury. Arch Phys Med Rehabil. 1993 Oct;74(10):1121-2.
- ↑ Bolin I, Bodin P, Kreuter M. Sitting position - posture and performance in C5 - C6 tetraplegia. Spinal Cord. 2000 Jul;38(7):425-34.
- ↑ Taricco M, Pagliacci MC, Telaro E, Adone R. Pharmacological interventions for spasticity following spinal cord injury: results of a Cochrane systematic review. Eura Medicophys. 2006 Mar;42(1):5-15.
- ↑ Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010 Dec 24;70(18):2409-38.
- ↑ Hashemi M, Sturbois-Nachef N, Keenan MA, Winston P. Surgical Approaches to Upper Limb Spasticity in Adult Patients: A Literature Review. Front Rehabil Sci. 2021 Aug 31;2:709969.
- ↑ Eltorai I, Montroy R. Muscle release in managing spasticity in spinal cord injury. Paraplegia. 1990 Sep;28(7):433-40.