Case Study - Myotonic Dystrophy Type 1
Original Editor -Michael Miglietta, Queen's University Neuromotor Function Project - Physiopedia (physio-pedia.com)
Top Contributors - Sarah Medeiros, Sienna De Caro, Kaylyn Turcotte
Abstract[edit | edit source]
This fictional case study involves 30-year-old Mr. J diagnosed with Myotonic Dystrophy Type 1 6 years ago and is currently presenting with global muscular weakness. Mr. J was referred to Physical Therapy to improve mobility and manage effects of his progressive disorder. Mr. J and the physical therapy team worked together to create patient-centred goals to guide his treatment plan. Interventions included: diaphragmatic breathing; muscle strengthening; balance, coordination and gait training; and orthotics and mobility aids. After Mr. J’s participation in Physical Therapy, he demonstrated improvements in muscular strength, gait speed, and balance, allowing him to successfully ambulate and participate within the community. Mr. J will continue to attend Physical Therapy sessions and be referred to a speech language pathologist and occupational therapist for a home assessment. Despite the significant improvements in Mr. J’s functional capacity, he still expresses concerns regarding his progressive condition, indicating that he may also benefit from appointments with a social worker.
Introduction[edit | edit source]
Myotonic dystrophy (DM) is a rare progressive disease[1] with multisystem effects seen in the muscles and other body systems[2]. It is also very common for patients with DM to present with cardiac conduction defects and cataracts[1]. DM is considered to be a subgroup of myopathies [1] which refers to a variety of diseases which primarily affect the skeletal muscle and lead to muscular weakness[3]. Although physiotherapy cannot assist in reversal or cure of this disease, it can help to manage and slow progression of the disease, along with minimize possible comorbidities and secondary effects[4].
DM affects approximately every 1 in 8,000 people, making it the most common adult-onset muscular dystrophy[5], despite the disease itself being quite rare. DM is often diagnosed through physical and subjective examinations performed by a Physician[6]. Additionally, since patients with DM often present with cataracts, an optometrist may be able to recognize this and refer the patient to a specialist for further examination and diagnosis[6]. Diagnoses can be confirmed through genetic DNA testing, to confirm whether the genes associated with DM are present[6].
DM is subdivided into two types:
Type 1 Myotonic Dystrophy (DM1) also known as Steinert disease[1] affects both skeletal and smooth muscle, along with the eyes, heart, endocrine system and central nervous system[7]. DM1 is the more common type seen in individuals with DM[8]. DM1 is caused by “expansion of the CTG repeat in the noncoding region of DMPK”[7] with a CTG repeat of greater than 34 repeats in length being considered abnormal[7]. DM1 is further divided into one of three phenotypes including: mild DM1, classic DM1, and congenital DM1, each presenting with different symptoms.
Signs/symptoms of Mild DM1 include: cataracts, and mild myotonia, with a normal life span[7]
Signs/symptoms of Classic DM1 include: muscle weakness and wasting, myotonia, cataracts, cardiac conduction abnormalities, decreased physical capacity, and potential decreased life span[7]
Signs/symptoms of Congenital DM1 include: hypotonia, severe generalized weakness at birth, respiratory insufficiency, intellectual disability and decreased life span[7]
Type 2 Myotonic Dystrophy (DM2) is the less common of the two subtypes. Patients with DM2 present with myotonia and muscle dysfunction[9], similar to DM1; however, effects of other body systems, such as cataracts and cardiac deficits are less commonly seen[9]. DM2 is caused by the “expansion of cytosine-cytosine-thymine-guanine (CCTG) tetranucleotide repeat located in the intron of the CCHC-type zinc finger nucleic acid-binding protein (CNB or ZNF9) gene or chromosome 3q21.3” [1].
Several studies have supported Physiotherapy as a treatment intervention for individuals with DM1. It has been reported that muscular strength can increase in patients with DM1 when provided with strength training programs [10][11][12]. Specifically, Roussel, Hebert & Duschesne (2020) followed 11 men with DM1 who completed a 12-week lower extremity strengthening program. When compared to baseline, performance in both strength and functional tests improved significantly[12] indicating that this is an effective intervention in the scope of Physiotherapy. Similarly, Missaoui et al. (2010) examined the effectiveness of a rehabilitation program in patients with DM, and found significant improvements in balance (measured by the Berg Balance Scale), gait speed and muscle strength[13]. Evidence supports the importance of Physiotherapy in management of DM, which is why it is necessary for Physiotherapists to have a strong understanding of the disease, clinical presentation, and possible treatment interventions.
In this fictional case, we will be following the treatment of 30-year-old male, Mr. J who has been diagnosed with DM1 and referred to Physiotherapy by his physician. Mr. J initially came to Physiotherapy presenting with severe musculoskeletal weakness, decreased cardiovascular and respiratory function, which overall affected his ability to ambulate and participate in activities of daily living. This fictional case will discuss the initial assessment, outcome measures, treatment interventions and outcomes throughout Mr. J’s time in Physiotherapy.
The objective of this fictional case is to provide Physiotherapists a resource on how to recognize the clinical presentation of DM1 and provide information on possible treatment interventions and outcome measures.
Client Characteristics[edit | edit source]
The patient in which this case study will be revolving around is Mr. J. Mr. J is a 30-year-old male who was diagnosed with myotonic dystrophy Type 1 (DM1) six year ago. Myotonic Dystrophy Type 1 is an autosomal dominant condition that has a multi-system effect (Thornton, 2014). Mr. J lives with his girlfriend in their bungalow and helps her with her online business, mostly answering calls as customer support. Mr. J was referred to physical therapy by his family doctor to help improve his level of mobility and to try to attenuate some of the negative effects of his progressive condition. Mr. J has no other significant comorbidities.
Examination Findings[edit | edit source]
Upon assessment, it was clear that Mr. J had mobility difficulties. Mr. J entered the clinic with the use of a single point cane (which he placed in his right hand) but also required the assistance of his girlfriend on his other hand. Mr. J requested his girlfriend be present for the duration of the assessment.
Prior to coming to the appointment, Mr. J completed the Assessment of Life Habits 3.1 short-form questionnaire which was sent to him by our clinic since this is a self-reported measure been validated for use in myotonic dystrophy patients (Assessment of Life Habits). Mr. J’s score was 7 and in particular his mobility score was 6.
Once Mr. J and his girlfriend were situated in one of our patient rooms, I conducted Mr. J subjective assessment. Mr. J noted a consistent and steady decline in his mobility status since being diagnosed with his condition, particularly noticing the effort it takes to walk being the most drastic change. Mr. J noted he is having difficulty coordinating his feet and ankles, reporting “sometimes my ankles don’t move the way I want them to which is affecting my balance”. He also has a hard time completing his ADLs without the help of his girlfriend and notices that within the last year, his breathing has become “a lot more labored”. When asked what he hopes to get out of physical therapy, he responded with “be able to walk from bedroom to kitchen without stopping at his desk to take a break in the morning and to complete more daily activities independently.”
Clinical Hypothesis[edit | edit source]
Diagnosis :
Myotonic dystrophy is medically diagnosed through genetic testing. However, the physical therapy diagnosis for Mr. J would be, “patient presents with impaired motor function of the distal upper and lower extremities bilaterally, dropped head, impaired balance, apical breathing pattern, and requires moderate assistance for completion of ADLs”
Problem list:
1. Muscle atrophy in distal upper and lower extremities due to disuse
2. Muscle weakness in distal upper and lower extremities due to disuse
3. Decrease upper extremity coordination due to underlying neurological impairment
4. Drop foot gait pattern throughout swing phase due to dorsiflexor weakness bilaterally
5. Apical breathing pattern due to weakened diaphragm
6. Dropped head presentation due to weak neck extensors
7. Impaired balance due to coordination impairment and muscular weakness
8. Increased fatigability due to sedentary lifestyle
9. Impaired muscle relaxation post-contraction due to underlying neurological impairment
Intervention[edit | edit source]
Patient centered goals[edit | edit source]
As Mr. J previous indicated his goals of physical therapy were to, “be able to walk from bedroom to kitchen without stopping at his desk to take a break in the morning and to complete more daily activities independently.”
When taking these goals as outlined by Mr. J, the following long-term goals were created:
- Prior to discharge from physical therapy, the patient will be able to achieve a 2-point increase in their score regarding mobility on the Assessment of Life Habits as compared to their initial assessment
- Prior to discharge from physical therapy, the patient will be able to achieve a 0.8m/s gait speed score on their 10m walk test
- Prior to discharge from physical therapy, the patient will be able to dress themselves with minimal assistance (no more than 3 points of contact) from the physical therapist
- Prior to discharge, patient will be able to walk from his bedroom to his kitchen without having to take a break
Once these long-term goals were identified and agreed upon by Mr. J, the following short-term goals were created to meet the long-term goals above:
- After two weeks, the patient will be able to demonstrate an increase in neck extension MMT from a 3-/5 to a 4-/5 when assessed for a single activation.
- After two weeks, the patient will be able to demonstrate a peak cough flow equal to or greater than 300L/min
- After three weeks, the patient will demonstrate an increase in finger flexion strength by increasing hand dynamometer strength by 5 kilograms for each hand.
- After three weeks, the patient will improve ankle dorsiflexion MMT from a 2-/5 to a 3/5 when assessed for a single activation.
- After three weeks, the patient will be able to demonstrate an increase in their 30 second sit-to-stand result by 3 repetitions with the use of a chair’s armrest
- After four weeks, the patient will show a decrease his time to complete the nine hole peg test of 3 seconds for his non-dominant hand and 4 seconds for his dominant hand.
- After four weeks, the patient will demonstrate an increase in their Berg Balance Scale from 43/56 points to 48/56 points
It is critical to remember that during goal setting, the patient is heavily involved in this process and must have their voice always heard.
Treatment Plan[edit | edit source]
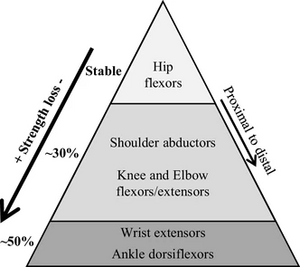
When planning intervention strategies to meet the goals above, it is important to remember that DM1 is a progressive neuromuscular disorder. This means that although it is possible for patients who have no experience with physical therapy to show improvements over short periods of time, over a long term (several years), a patient's conditions will worsen naturally. For example, in one study, it was found that over nine years 30.3-43.5% of individuals with DM1 presented with loss of muscle strength as compared to individuals without DM1 (Gagnon et al. 2019). Therefore, it is the responsibility of physical therapist to help attenuate the consequences of such a progressive disorder as best as possible.
Interventions provided to Mr. J were considered in tandem with Mr. J to ensure that he was an active participant in this process.
The following interventions were provided:
Diaphragmatic breathing- to improve diaphragmatic breathing, patient facilitated tactile cued deep breathing was conducted for ten repetitions every waking hour. Such an intervention was used to target Mr. J's apical breathing pattern and to ensure that Mr. J is taking deep enough breaths to help facilitate productive coughs if required. Also, preliminary research in healthy individuals shows the potential of diaphragmatic breathing as being associated with improvements in balance as well (Stephens et al. 2017). Therefore, Mr. J may also potential receive this add benefit from such an intervention as well.
Strengthening- exercises were provided targeting the initial limitations noted in the assessment which include: finger flexion weakness, ankle dorsiflexion weakness, and neck extensor weakness. The exercises were prescribed under parameters that were identified as being safe for patients with DM1 to perform (Exercise Guide for People Living with Myotonic Dystrophy) and were completed to muscular fatigue, not muscular failure. Exercises prescribed can be found in the table below (Table 1) and target the muscle groups that were observed as being weak in Mr. J's assessment. In addition, isotonic exercises were avoided to prevent any exacerbation of Mr. J's underlying myotonia. However, quarter squats and light weight over-head shoulder press exercises were also provided to Mr. J under the same parameters to help improve his general fatigability and general muscular strength. In the literature there seems to be the possibility of those with DM1 to present with skeletal muscle growth (Roussel et al. 2019) and that strength in DM1 patients can be increased when following an exercise program (Brady et al. 2014). As Mr. J progressed through his therapy interventions, the following exercises were also progressed to more difficult variations.
| Type | Intensity | Reps | Sets | Rest between sets | Times per week |
| Seated ankle dorsiflexion | Moderate (60% 1RM) | 8-12 |
|
|
2-3 |
| Prone Knee extensions | |||||
| Mass finger flexion against ball | |||||
| Body weight quarter squats | |||||
| Light weight over-head shoulder press |
Table 1: strengthening exercises prescription and parameters provided to Mr. J
Muscular relaxation- Mr. J was educated on the what his underlying myotonia meant and referred back to his physician if his current medication no longer helped.
Balance- patients with DM1 are seen as similar falls risks to elderly individuals (de Die-Smulders CE, 1998) and have impaired ankle strategy usage when balance is lost due to weakness of the ankle musculature (Exercise Guide for People Living with Myotonic Dystrophy). Also, the drop foot that a person with DM1 presents with puts them at an increased risk of tripping when walking (Exercise Guide for People Living with Myotonic Dystrophy). Therefore, the dorsiflexion strengthening exercises as listed above may improve balance as well, but balance exercises were also provided to Mr. J. Based on Mr. J’s presentation, a prescription of narrow stance static standing holds for ten seconds for ten repetitions daily were provided. As Mr. J's balance improved, exercises progressed to more challenging interventions (tandem stance) and then to more mobile and task-specific interventions (reaching outside base of support). However, given Mr. J's falls risk, such exercises were completed in parallel bars and under contact supervision of the therapist. Recent research has also suggested that post-balance intervention with DM1 patients is likely to result in a self-reported increase in balance confidence (Hammarén et al. 2015).
Coordination- In order to improve Mr. J’s coordination, task-specific interventions were used. These interventions included tasks that Mr. J deemed meaningful such as: typing, washing the dishes, dressing himself, and being able to open the drawers in his house. Coordination exercises were initially blocked prior to progressing to more random practice as Mr. J improved. In addition, time was provided to Mr. J after each task for self-reflection and to allow Mr. J to problem solve as well as to identify any errors in his task completion prior to him receiving feedback of the results from the physical therapist. However, thumb and finger tapping were also provided to Mr. J for at home completion.
Gait training- gait training was a substantial portion of Mr. J’s therapeutic intervention. However, for such a condition, therapists used a bandwidth feedback approach and allowed the patient time to self-reflect prior to providing feedback on the knowledge of results. Mr. J also completed body weight supported treadmill training in a closed environment in order to help facilitate more coordinated gait movements prior to being progressed to gait training in more open environments with great variability (without a balance system) to provide more generalizability to his life environment and to increase his endurance.
Orthotics/Gait aids - however, given the progressive nature of the condition, Mr. J was placed in an AFO to help address his foot drop as well as being properly fit, educated, and taught how to use a 2 wheeled walker. Both changes were implemented to help improve Mr. J’s stability while ambulating. An Occupational Therapist (OT) was consulted in order to ensure all aids provided were appropriate for Mr. J’s condition.
In addition, a referral was also made to a Speech Language Pathologist to help target Mr. J’s facial and oral musculature weakness. Throughout the intervention process, constant education was provided to Mr. J regarding several concepts. Some of these concepts included: his condition, the reason why intervention techniques were chosen, any potential risks or benefits from a given intervention, any alternative intervention strategies, and how DM1 may progress and continue to affect his mobility. The goal of this was to ensure Mr. J properly understood the therapy being provided to him and to properly prepare him for what may be his future reality.
Outcome[edit | edit source]
Grip strength improved slightly: R 29.1kg L 26.9kg
30 second sit-to-stand test improved to 15 full repetitions
10m walk test improved to 0.78m/s which is just below the 0.8m/s cut-off required for safe community ambulation
Peak cough flow increased to 310L/min.
Nine hole peg test- right hand: 16.73s, left hand: 18.12s
BERG Balance Scale score improved to 48/56 which indicates that his balance meets the score to be able to ambulate without a mobility aid. However, this improvement in score is also highly attributed to the improvements in strength of the lower extremity which allowed for better control of motor movements when completing dynamic items in this test such as, sit-to-stand and transfers. Therefore, Mr. J will continue to use a mobility aid (2 wheel-walker) when ambulating longer distance. This improvement was also attributed to the balance interventions that improved his ankle strategy.
10mWT improved to 0.78m/s, which is a speed that places Mr. J as a potential community ambulator. This improvement was due to a combination of improved aerobic capacity, strengthening of the respiratory muscles and learned deep breathing techniques. Although his lower extremity strength improved, he still had difficulty ambulating for long periods due to ankle dorsiflexor fatigue, though Mr. J was able to ambulate from his bedroom to his kitchen with the use of an AFO.
The discharge planning includes treatment techniques and referrals to specialists to reduce the long-term changes that will occur with DM1. Since it is a progressive disorder affecting multiple body systems, it requires a multidisciplinary treatment and management approach (Portaro, et al., 2017). After the 6-week treatment intervention, Mr. J will continue to receive physiotherapy treatment once per week to monitor the disease progression and maintain the benefits gained from the treatment program. As noted in the initial observation, Mr. J exhibited signs of facial and oral muscle weakness. Therefore, referral to a speech language therapist would be beneficial to assist in the activation and maintenance of the strength, endurance, and coordination of those muscles (Harper, Van Engelen, Eymard, Rogers, & Wilcox, 2002). As well, as the condition progresses, Mr. J may experience difficulty swallowing and eating so this would help them learn effective strategies to do so safely and effectively. Along with physiotherapy biweekly, to slow the progression of muscle atrophy, Mr. J will be referred to a community-based exercise program close to his home, where he can continue to do light to moderate strength and endurance exercise 3-5 days per week which will also benefit his cardiorespiratory system function. The weakening of respiratory muscles results in symptoms of dyspnea, so Mr. J will be educated by the physiotherapist about self-management positions he can do to relieve these symptoms. This includes sleeping in side-lying and upper body elevation as well as the tripod position when sitting. Before returning home, the occupational therapist will assess the safety of Mr. J’s home and make suggestions for any adaptations that would improve his mobility and ability to be independent in his activities of daily living (i.e. grab bars in the bathroom, removal of carpets) (Harper, et al., 2002).
Approaching the end of his 6-week intervention, Mr. J was feeling more confident in his community ambulation and slightly stronger. He was grateful for the knowledge he learned about his condition and the things that could be done to slow its progression, however, he still expressed worry about the effect it would have on his quality of life in the future, Therefore, Mr. J will also benefit from a referral to a social worker to help him cope and remain socially integrated (Harper, et al., 2002).
Discussion[edit | edit source]
Type 1 Myotonic Dystrophy (DM1) affects both skeletal and smooth muscle, along with the eyes, heart, endocrine system and central nervous system (7). Signs and symptoms can include muscle weakness and wasting, myotonia, cardiac conduction abnormalities, decreased physical capacity, and potential decreased life span (7). Fortunately, several studies have supported Physiotherapy treatment interventions and interdisciplinary care for individuals with DM1. Dysphagia is a common symptom of DM1, Parkinson’s disease and multiple sclerosis, therefore, the intervention techniques in this study for facial muscle strengthening and coordination, along with referral to a speech language therapist (Molokwu, 2015). Mr. J is one of the many people who, through rehab and education, focused on task-specific and functional activities meaningful to his daily life and was able to slow the progression of his disease and gain confidence. Physiotherapists play a crucial role in preventing progression, but also in teaching compensatory approaches to maintain patient functional independence and participation. Physiotherapists also play a large role in communication with other health care providers, the patient and the patient's family/ caregivers. With the use of diaphragmatic breathing and lower extremity strengthening, and evidence of assistive devices (AFO, 2WW), Mr. J was able to achieve most of his goals. The development of rapport with patients combined with collaborative goal setting and treatment planning using the International Classification of Functioning, Disability and Health (ICF model), physiotherapists can help people with DM1 reach their goals. Ultimately, this influences their quality of life and outlook on living with a progressive disease.
Self Study Questions[edit | edit source]
1. Which standardized outcome measures are reliable and valid for DM1 patients?
A: Assessment of Life Habits
B: 30 second sit to stand test
C: 10-meter walk test
D: Berg Balance Scale
E: All the above
(Answer E)
2. Is it possible for individuals with DM1 to improve their strength and present with skeletal muscle growth
A: False
B: True
(Answer: B)
3. As DM1 is a progressive neuromuscular condition, patients diagnosed with the condition will not benefit from physical therapy treatment.
A: False
B: True
(Answer: A)
For further information about the purpose of this case study, please investigate the following Physiopedia page Queen's University Neuromotor Function Project - Physiopedia (physio-pedia.com)
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 Vydra DG, Rayi A. Myotonic dystrophy. InStatPearls [Internet] 2021 Feb 11. StatPearls Publishing.
- ↑ Myotonic dystrophy - about the disease [Internet]. Genetic and Rare Diseases Information Center. U.S. Department of Health and Human Services; [cited 2022May11]. Available from: https://rarediseases.info.nih.gov/diseases/10419/myotonic-dystrophy
- ↑ Myopathy: Causes, symptoms, diagnosis & treatment [Internet]. Cleveland Clinic. [cited 2022May11]. Available from: https://my.clevelandclinic.org/health/diseases/17256-myopathy
- ↑ Duong T, Eichinger K. Role of physical therapy in the assessment and management of individuals with myotonic dystrophy [Internet]. myotonic.org. [cited 2022May11]. Available from: https://www.myotonic.org/sites/default/files/pages/files/MDF_RoleofPhysicalTherapy_1_21.pdf
- ↑ Suominen T, Bachinski LL, Auvinen S, Hackman P, Baggerly KA, Angelini C, Peltonen L, Krahe R, Udd B. Population frequency of myotonic dystrophy: higher than expected frequency of myotonic dystrophy type 2 (DM2) mutation in Finland. European journal of human genetics. 2011 Jul;19(7):776-82.
- ↑ 6.0 6.1 6.2 Diagnosis - myotonic dystrophy (DM) - diseases [Internet]. Muscular Dystrophy Association. 2021 [cited 2022May11]. Available from: https://www.mda.org/disease/myotonic-dystrophy/diagnosis
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Bird TD. Myotonic dystrophy type 1 - genereviews® - NCBI bookshelf [Internet]. [cited 2022May11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1165/
- ↑ Myotonic dystrophy: Medlineplus Genetics [Internet]. MedlinePlus. U.S. National Library of Medicine; [cited 2022May11]. Available from: https://medlineplus.gov/genetics/condition/myotonic-dystrophy/#frequency
- ↑ 9.0 9.1 Schoser B. Myotonic Dystrophy Type 2 [Internet]. [cited 2022May11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1466/
- ↑ Roussel MP, Morin M, Girardin M, Fortin AM, Leone M, Mathieu J, Gagnon C, Duchesne E. Training program-induced skeletal muscle adaptations in two men with myotonic dystrophy type 1. BMC Research Notes. 2019 Dec;12(1):1-6.
- ↑ Brady LI, MacNeil LG, Tarnopolsky MA. Impact of habitual exercise on the strength of individuals with myotonic dystrophy type 1. American journal of physical medicine & rehabilitation. 2014 Sep 1;93(9):739-50.
- ↑ 12.0 12.1 Roussel MP, Hébert LJ, Duchesne E. Strength-training effectively alleviates skeletal muscle impairments in myotonic dystrophy type 1. Neuromuscular Disorders. 2020 Apr 1;30(4):283-93.
- ↑ Missaoui B, Rakotovao E, Bendaya S, Mane M, Pichon B, Faucher M, Thoumie P. Posture and gait abilities in patients with myotonic dystrophy (Steinert disease). Evaluation on the short-term of a rehabilitation program. Annals of physical and rehabilitation medicine. 2010 Aug 1;53(6-7):387-98.