Proteoglycans: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
== Introduction == | == Introduction == | ||
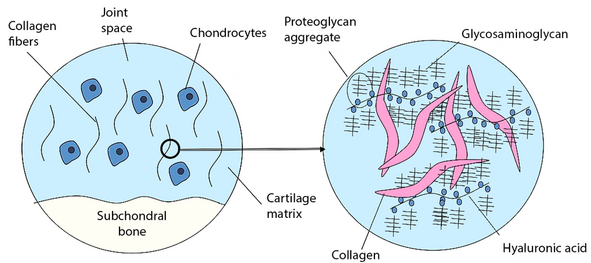
[[File:Glycosaminoglycans.png|thumb|591x591px|GAGs, proteoglycan, glycoproteins, cartilage matrix]] | [[File:Glycosaminoglycans.png|thumb|591x591px|GAGs, proteoglycan, glycoproteins, cartilage matrix]] | ||
Proteoglycan are of a class of glycoproteins of high molecular weight that are found especially in the extracellular matrix of connective tissue (the fibrous tissue that gives support to the body structure). <ref>Merriam Webster Proteoglcan Available:https://www.merriam-webster.com/dictionary/proteoglycan (accessed 18.6.2022)</ref> Proteoglycans make up a major part of the extracellular matrix, filling the spaces that occur between cells. Different from other body tissues, the extracellular matrix is the most significant part of connective tissue.<ref name=":0">All the science Proteoglycans Available: https://www.allthescience.org/what-are-proteoglycans.htm (accessed 18.6.2022)</ref> | |||
== Classification == | == Classification == | ||
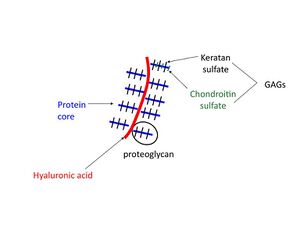
[[File: | [[File:Glycosaminoglycans2.jpeg|thumb|GAGs, proteoglycan, glycoprotein, keratan sulfate, cartilage matrix]] | ||
Ways to classify | |||
# Nature of their GAGs chains: Proteoglycans can be classified according to the glycosaminoglycans (GAGs) attached to them. The types are chondroitin sulfate, dermatan sulfate, heparin sulfate, heparan sulfate, or keratan sulfate. | # Nature of their GAGs chains: Proteoglycans can be classified according to the glycosaminoglycans (GAGs) attached to them. The types are chondroitin sulfate, dermatan sulfate, heparin sulfate, heparan sulfate, or keratan sulfate. | ||
# | # Proteoglycans are categorized by their relative size (large and small): Large molecules e.g. aggrecan, an important part of cartilage, versican, which is found in the blood vessels and skin. Small molecules eg decorin, biglycan, fibromodulin, and lumican<ref name=":0" />. | ||
== Synthesis == | == Synthesis == | ||
Proteoglycans are synthesized in the rough endoplasmic reticulum | Proteoglycans are synthesized in the rough endoplasmic reticulum, then reshaped in the golgi body, and are ultimately transported to the extracellular matrix by vesicles.<ref name=":1">Lamoureux F, Baud'huin M, Duplomb L, Heymann D, Rédini F. Proteoglycans: key partners in bone cell biology. Bioessays. 2007 Aug;29(8):758-71. Avcailable:https://pubmed.ncbi.nlm.nih.gov/17621645/ (accessed 18.6.2022)</ref> | ||
== Diverse Functions == | == Diverse Functions == | ||
| Line 23: | Line 23: | ||
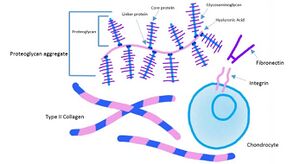
Proteoglycans are a major component of the animal extracellular matrix, being the filler between cells in an organism. Proteoglycans have an important role in the physiology and biomechanical function of [[Tendon Anatomy|tendons,]] [[Ligament|ligaments]] and [[Cardiovascular System|cardiovascular system]] via their involvement in regulation of assembly and maintenance of extracellular matrix, and as they participate in cell proliferation through their interactions with growth factors<ref>Halper J. Proteoglycans and diseases of soft tissues. Progress in heritable soft connective tissue diseases. 2014:49-58.Available:https://pubmed.ncbi.nlm.nih.gov/24443020/ (accessed 18.6.2022)</ref>. Eg | Proteoglycans are a major component of the animal extracellular matrix, being the filler between cells in an organism. Proteoglycans have an important role in the physiology and biomechanical function of [[Tendon Anatomy|tendons,]] [[Ligament|ligaments]] and [[Cardiovascular System|cardiovascular system]] via their involvement in regulation of assembly and maintenance of extracellular matrix, and as they participate in cell proliferation through their interactions with growth factors<ref>Halper J. Proteoglycans and diseases of soft tissues. Progress in heritable soft connective tissue diseases. 2014:49-58.Available:https://pubmed.ncbi.nlm.nih.gov/24443020/ (accessed 18.6.2022)</ref>. Eg | ||
* [[Cartilage]] contains a variety of proteoglycans, being essential for its normal function. These include aggrecan, decorin, biglycan, fibromodulin and lumican. Each proteoglycan serves several functions that are determinedby both its core protein and its glycosaminoglycan chains<ref>Carney SL, Muir H. The structure and function of cartilage proteoglycans. Physiological reviews. 1988 Jul;68(3):858-910. Available:https://www.researchgate.net/publication/19863408_The_structure_and_function_of_cartilage_proteoglycans (accessed 18.6.2022)</ref>. eg Aggrecan provides cartilage with the property to bind with water to form hydrated matrices. These molecules act as fillers between the cell spaces<ref name=":2">Biology wise A Concise Overview: Structure and Functions of Proteoglycans Available:https://biologywise.com/structure-function-of-proteoglycans (accessed 18.6.2022)</ref>. | * [[Cartilage]] contains a variety of proteoglycans, being essential for its normal function. These include aggrecan, decorin, biglycan, fibromodulin and lumican. Each proteoglycan serves several functions that are determinedby both its core protein and its glycosaminoglycan chains<ref>Carney SL, Muir H. The structure and function of cartilage proteoglycans. Physiological reviews. 1988 Jul;68(3):858-910. Available:https://www.researchgate.net/publication/19863408_The_structure_and_function_of_cartilage_proteoglycans (accessed 18.6.2022)</ref>. eg Aggrecan provides cartilage with the property to bind with water to form hydrated matrices. These molecules act as fillers between the cell spaces<ref name=":2">Biology wise A Concise Overview: Structure and Functions of Proteoglycans Available:https://biologywise.com/structure-function-of-proteoglycans (accessed 18.6.2022)</ref>. | ||
* Proteoglycans play important roles in organizing the [[bone]] extracellular matrix, taking part in the structuring of the tissue itself as active regulators of [[collagen]] fibrillogenesis.<ref name=":1" />The bone matrix has a lower proteoglycan content than those in the cartilage | * Proteoglycans play important roles in organizing the [[bone]] extracellular matrix, taking part in the structuring of the tissue itself as active regulators of [[collagen]] fibrillogenesis.<ref name=":1" />The bone matrix has a lower proteoglycan content than those in the cartilage and hence takes up less water as is more brittle<ref name=":2" />. | ||
== Dysfunction == | == Dysfunction == | ||
An inability to break down the proteoglycans (due to absent or malfunctioning lysosomal | An inability to break down the proteoglycans (due to absent or malfunctioning lysosomal enzymes) is characteristic of a group of genetic disorders, called mucopolysaccharidoses. Over time, these GAGs collect in the cells, blood and connective tissues. The result is permanent, progressive cellular damage which affects appearance, physical abilities, organ and system functioning.<ref name=":2" /> | ||
== older adult and Extracellular Matrix (proteoglycan) == | |||
Forever we want to be young! but our physiological systems do not allow. Integumentary system that play vital role in outward appearance and seat of many receptors become aged as a result of the ageing process. Glycosaminoglycans-- named proteoglycans when attached to a protein core are part of the extracellular matrix--decrease as we age and this is contributing to why human skin is ageing. And reduction or absence of sexual hormonal changes such as oestrogen has been noted to contribute to a reduction in Glycosaminoglycans and proteoglycans among older adults. | |||
Several GAGs and PGs in the skin may explain ageing skin. In intrinsic aged skin as well as photoaging skin, extracellular matrix components such as reduction in epidermal hyaluronan, versican, elastotic materials and dermal biglycan are related to why we have ageing skin. It is of expert opinion that analyzing the involvement of proteoglycans and glycosaminoglycans in addition to hormonal deficiency such as a drop in the level of oestrogen or complete absence in postmenopausal women may lead us to anti-ageing skin remedies. | |||
== References == | == References == | ||
Revision as of 07:23, 29 June 2022
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton and Tolulope Adeniji
Introduction[edit | edit source]
Proteoglycan are of a class of glycoproteins of high molecular weight that are found especially in the extracellular matrix of connective tissue (the fibrous tissue that gives support to the body structure). [1] Proteoglycans make up a major part of the extracellular matrix, filling the spaces that occur between cells. Different from other body tissues, the extracellular matrix is the most significant part of connective tissue.[2]
Classification[edit | edit source]
Ways to classify
- Nature of their GAGs chains: Proteoglycans can be classified according to the glycosaminoglycans (GAGs) attached to them. The types are chondroitin sulfate, dermatan sulfate, heparin sulfate, heparan sulfate, or keratan sulfate.
- Proteoglycans are categorized by their relative size (large and small): Large molecules e.g. aggrecan, an important part of cartilage, versican, which is found in the blood vessels and skin. Small molecules eg decorin, biglycan, fibromodulin, and lumican[2].
Synthesis[edit | edit source]
Proteoglycans are synthesized in the rough endoplasmic reticulum, then reshaped in the golgi body, and are ultimately transported to the extracellular matrix by vesicles.[3]
Diverse Functions[edit | edit source]
Proteoglycans are a major component of the animal extracellular matrix, being the filler between cells in an organism. Proteoglycans have an important role in the physiology and biomechanical function of tendons, ligaments and cardiovascular system via their involvement in regulation of assembly and maintenance of extracellular matrix, and as they participate in cell proliferation through their interactions with growth factors[4]. Eg
- Cartilage contains a variety of proteoglycans, being essential for its normal function. These include aggrecan, decorin, biglycan, fibromodulin and lumican. Each proteoglycan serves several functions that are determinedby both its core protein and its glycosaminoglycan chains[5]. eg Aggrecan provides cartilage with the property to bind with water to form hydrated matrices. These molecules act as fillers between the cell spaces[6].
- Proteoglycans play important roles in organizing the bone extracellular matrix, taking part in the structuring of the tissue itself as active regulators of collagen fibrillogenesis.[3]The bone matrix has a lower proteoglycan content than those in the cartilage and hence takes up less water as is more brittle[6].
Dysfunction[edit | edit source]
An inability to break down the proteoglycans (due to absent or malfunctioning lysosomal enzymes) is characteristic of a group of genetic disorders, called mucopolysaccharidoses. Over time, these GAGs collect in the cells, blood and connective tissues. The result is permanent, progressive cellular damage which affects appearance, physical abilities, organ and system functioning.[6]
older adult and Extracellular Matrix (proteoglycan)[edit | edit source]
Forever we want to be young! but our physiological systems do not allow. Integumentary system that play vital role in outward appearance and seat of many receptors become aged as a result of the ageing process. Glycosaminoglycans-- named proteoglycans when attached to a protein core are part of the extracellular matrix--decrease as we age and this is contributing to why human skin is ageing. And reduction or absence of sexual hormonal changes such as oestrogen has been noted to contribute to a reduction in Glycosaminoglycans and proteoglycans among older adults. Several GAGs and PGs in the skin may explain ageing skin. In intrinsic aged skin as well as photoaging skin, extracellular matrix components such as reduction in epidermal hyaluronan, versican, elastotic materials and dermal biglycan are related to why we have ageing skin. It is of expert opinion that analyzing the involvement of proteoglycans and glycosaminoglycans in addition to hormonal deficiency such as a drop in the level of oestrogen or complete absence in postmenopausal women may lead us to anti-ageing skin remedies.
References[edit | edit source]
- ↑ Merriam Webster Proteoglcan Available:https://www.merriam-webster.com/dictionary/proteoglycan (accessed 18.6.2022)
- ↑ 2.0 2.1 All the science Proteoglycans Available: https://www.allthescience.org/what-are-proteoglycans.htm (accessed 18.6.2022)
- ↑ 3.0 3.1 Lamoureux F, Baud'huin M, Duplomb L, Heymann D, Rédini F. Proteoglycans: key partners in bone cell biology. Bioessays. 2007 Aug;29(8):758-71. Avcailable:https://pubmed.ncbi.nlm.nih.gov/17621645/ (accessed 18.6.2022)
- ↑ Halper J. Proteoglycans and diseases of soft tissues. Progress in heritable soft connective tissue diseases. 2014:49-58.Available:https://pubmed.ncbi.nlm.nih.gov/24443020/ (accessed 18.6.2022)
- ↑ Carney SL, Muir H. The structure and function of cartilage proteoglycans. Physiological reviews. 1988 Jul;68(3):858-910. Available:https://www.researchgate.net/publication/19863408_The_structure_and_function_of_cartilage_proteoglycans (accessed 18.6.2022)
- ↑ 6.0 6.1 6.2 Biology wise A Concise Overview: Structure and Functions of Proteoglycans Available:https://biologywise.com/structure-function-of-proteoglycans (accessed 18.6.2022)