Intervertebral disc
Definition/Description[edit | edit source]
The intervertebral disc (IVD) is important in the normal functioning of the spine. It is a cushion of fibrocartilage and the principal joint between two vertebrae in the spinal column. There are 23 discs in the human spine: 6 in the cervical region (neck), 12 in the thoracic region (middle back), and 5 in the lumbar region (lower back).
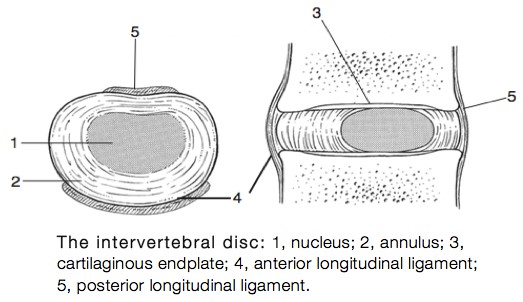
IVDs allow the spine to be flexible without sacrificing a great deal of strength. They also provide a shock-absorbing effect within the spine and prevent the vertebrae from grinding together. They consist of three major components: the inner, nucleus pulposus (NP), the outer, annulus (AF) and the cartilaginous endplates that anchor the discs to adjacent vertebrae.[1]
Clinically Relevant Anatomy[edit | edit source]
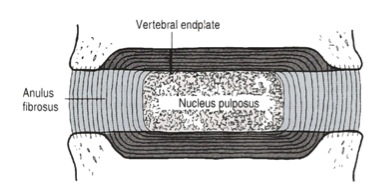
The IVD consists of three distinct components (Figure 2):
- A central nucleus pulposus (NP);
- A peripheral annulus fibrosus (AF);
- Two vertebral endplates (VEPs).
Figure 2: Detailed structure of the IVD (adapted from Bogduk 2005)
Nucleus Pulposus[edit | edit source]
A gel-like structure that sits at the center of the intervertebral disc and accounts for much of the strength and flexibility of the spine. It is made of 66% to 86% water with the remainder consisting of primarily type II collagen (it may also contain type VI, IX, and XI) and proteoglycans. The proteoglycans include the larger aggrecan and versican that bind to hyaluronic acid, as well as several small leucine-rich proteoglycans. Aggrecan is largely responsible for retaining water within the NP. This structure also contains a low density of cells. While sparse, these cells produce the extracellular matrix (ECM) products (aggrecan, type II collagen, etc.) and maintain the integrity of the NP.[1]
Annulus Fibrosus[edit | edit source]
Consists of “lamellae” or concentric layers of collagen fibres [2]. The fibre orientation of each layer of lamellae alternate and therefore allow effective resistance of multidirectional movements. The AF contains an inner and an outer portion. They differ primarily in their collagen composition. While both are primarily collagen, the outer annulus contains mostly type I collagen, while the inner has predominantly type II. The inner AF contains more proteoglycans compared to the outer AF.[3][1] NB collagen Type I: skin, tendon, vasculature, organs, bone (the main component of the organic part of bone) Type II: cartilage (the main collagenous component of cartilage and is more flexible)[4]
Vertebral Endplate[edit | edit source]
An upper and a lower cartilaginous endplate (each about 0.6– 1 mm thick) covers the superior and inferior aspects of the disc. The endplate permits diffusion and provides the main source of nutrition for the disc. The hyaline endplate is also the last part of the disc to wear through during severe disc degeneration.
- Plates of cartilage that bind the disc to their respective vertebral bodies.
- Each endplate covers almost the entire surface of the adjacent vertebral body; only a narrow rim of bone, called the ring apophysis, around the perimeter of the vertebral body, is left uncovered by cartilage.
- The portion of the vertebral body to which the cartilaginous endplate is applied is referred to as the vertebral endplate.
- The endplate covers the nucleus pulposus in its entirety; peripherally it fails to cover the entire extent of the annulus fibrosus.
- The collagen fibrils of the inner lamellae of the annulus enter the endplate and merge with it, resulting in all aspects of the nucleus being enclosed by a fibrous capsule.[5]
Innervation[edit | edit source]
The disc is innervated in the outer few millimetres of the annulus fibrosus [6].
Only the outer third of the AF is vascular and innervated in a non-pathologic state. In ageing and states of inflammation, both nerve growth and granulation tissue growth are stimulated. Additionally, the granulation tissue secretes inflammatory cytokines, which further increases sensitivity to pain sensations[1].
Vascular Supply and Nutrition[edit | edit source]
The IVD is largely avascular, with no major arterial branches to the disc [7]. The outer annular layers are supplied by small branches from metaphysical arteries. Only the outer annulus is vascularized. Blood vessels near the disc-bone junction of the vertebral body as well as those in the outer annulus supply the NP and inner annulus. Glucose, oxygen and other nutrients reach the avascular regions by diffusion. The same process removes metabolites.[1]
There is a scoping review[8] challenges the traditional view of intervertebral discs (IVDs) as completely bloodless tissue. While the nucleus pulposus remains avascular throughout life, the surrounding structures – the cartilage endplates and the annulus fibrosus – receive a significant blood supply early on. However, this blood supply decreases as we age. Additionally, damaged or disrupted disc tissue, regardless of age, often shows evidence of new blood vessel growth within the cartilage endplates and inner layers of the annulus fibrosus.[8]
Vital functions[edit | edit source]
- Restricted IV joint motion.
- Contribution to stability.
- Resistance to axial, rotational, and bending load.
- Preservation of anatomic relationship.
- It provides cushioning for the vertebrae and reduce the stress caused by impact.
- They act as shock absorber for the spine.
- They help protect the nerves that run down the spine and between the vertebrae.[9][9]
Biomechanics[edit | edit source]
Weight bearing: The disc is subjected to various loads, including compressive, tensile and shear stresses [10] [11]. During compressive loading, hydrostatic pressure develops within the NP, which thereby disperses the forces towards the endplates as well as the AF [12] [13] [12]. This mechanism slows the rate applied loads are transmitted to the adjacent vertebra, giving the disc its shock-absorbing abilities [14].
Movement: The disc is also involved in permitting movements between vertebral bodies, which include:
- Axial compression/distraction;
- Flexion / extension;
- Axial rotation;
- Lateral flexion.
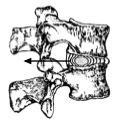
Nuclear migration: Asymmetric compressive loading disc can cause the NP to migrate in a direction opposite to the compression [15] [14] [16] [17]. For example, during forward bending (or flexion) of the lumbar spine, the NP migrates posteriorly or backwards (Figure 4). Conversely, during backwards bending (or extension), the nucleus is squeezed anteriorly or forward. This concept is known as the dynamic disc model [18]. Although NP migration has been shown to behave predictably in asymptomatic discs, a variable pattern of migration occurs in people with symptomatic and/or degenerative IVDs [18].
Figure 4: Direction of nuclear migration within the IVD during spinal movements (adapted from McKenzie 1981)
Physiologic Variants[edit | edit source]
Disc thickness generally increases from rostral to caudal. The thickness of the discs relative to the size of the vertebral bodies is highest in the cervical and lumbar regions. This reflects the increased range of motion found in those regions.
In the cervical and lumbar regions, the intervertebral discs are thicker anteriorly. This creates the secondary curvature of the spine – the cervical and lumbar lordoses.[1]
Pathology[edit | edit source]
There are several terms to describe disc pathologies
- Disc bulge ie the circumference of disc extends beyond the vertebral bodies.
- Disc herniation involves the NP. Disc herniation is significant in that it may compress an adjacent spinal nerve. A herniated disc impinges upon the nerve associated with the inferior vertebrae (e.g., L4/L5 herniation affects the L5 nerve root). The most common site of disc herniation is at L5-S1, which may be due to the thinning of the posterior longitudinal ligament towards its caudal end. There are three subtypes of herniations:
- Disc protrusion is characterized by the width of the base of the protrusion is wider than the diameter of the disc material that is herniated.
- In disc extrusion, the AF is damaged, allowing the NP to herniate beyond the normal bounds of the disc. In this case, the herniated material produces a mushroom-like dome that is wider than the neck connecting it to the body of the NP. The herniation may extend superior or inferiorly relative to the disc level.
- In disc sequestration, the herniated material breaks off from the body of the NP.
- Disc desiccation is common in aging. It is brought about by the death of the cells that produce and maintain the ECM, including proteoglycans, such as aggrecan. The NP shrinks as the gelatinous form is replaced with fibrotic tissue, reducing its functionality, and leaves the AF supporting additional weight. This increased stress leads the AF to compensate by increasing in size. The resulting flattened disc reduces mobility and may impinge on spinal nerves leading to pain and weakness. It is thought to be due to proteoglycan breakdown, which reduces the water-retaining properties of the NP.
NB: Significant research has been put into means of replacing/re-growing the intervertebral discs. The various methods include the replacement of discs with synthetic materials, stem cell therapy, and gene therapy.[1]
Additional Points[edit | edit source]
There is no intervertebral disc between C1 and C2, which is unique in the spine.
Two major ligaments support the intervertebral discs.
- The anterior longitudinal ligament is broadband that covers the anterolateral surface of the spine from the foramen magnum in the skull to the sacrum. This ligament assists the spine in preventing hyperextension and prevents intervertebral disc herniation in the anterolateral direction.
- The posterior longitudinal ligament covers the posterior aspect of the vertebral bodies, within the vertebral canal, and serves mainly to prevent a posterior herniation of the intervertebral discs, and is therefore responsible for the most herniations being in the postero-lateral direction.[1]
Related Pages[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Waxenbaum JA, Futterman B. Anatomy, Back, Intervertebral Discs. InStatPearls [Internet] 2018 Dec 13. StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470583/ (last accessed 27.1.2020)
- ↑ Marchand, F. and A.M. Ahmed, Investigation of the laminate structure of lumbar disc annulus fibrosus. Spine (Phila Pa 1976), 1990. 15(5): p. 402-10.
- ↑ Fearing BV, Hernandez PA, Setton LA, Chahine NO. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR spine. 2018 Sep;1(3):e1026. [1]
- ↑ Peptan Collagen Available from:https://www.peptan.com/collagen-in-our-bodies/ (last accessed 27.1.2020)
- ↑ Musculoskeletal key Applied anatomy of the lumbar spine Available from:https://musculoskeletalkey.com/applied-anatomy-of-the-lumbar-spine/ (last accessed 27.1.2020)
- ↑ Roberts, S., et al., Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am, 2006. 88 Suppl 2(Supplement 2): p. 10-4.
- ↑ Urban, J.P., et al., Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop Relat Res, 1977(129): p. 101-14.
- ↑ 8.0 8.1 Fournier DE, Kiser PK, Shoemaker JK, Battié MC, Séguin CA. Vascularization of the human intervertebral disc: a scoping review. JOR spine. 2020 Dec;3(4):e1123. [2]
- ↑ 9.0 9.1 Intervertebral disc. Study.com. Available from https://study.com/academy/lesson/intervertebral-disc-definition-function-disease.html [last accessed 13/10/2020]
- ↑ Stokes, I. and J. Iatridis, Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine (Phila Pa 1976), 2004. 29(23): p. 2724-32.
- ↑ White, A.A. and M.M. Panjabi, Clinical biomechanics of the spine. Vol. 446. 1990: Lippincott Philadelphia.
- ↑ 12.0 12.1 Reuber, M., et al., Bulging of lumbar intervertebral disks. J Biomech Eng, 1982. 104(3): p. 187-92.
- ↑ Broberg, K.B., On the mechanical behaviour of intervertebral discs. Spine (Phila Pa 1976), 1983. 8(2): p. 151-65.
- ↑ 14.0 14.1 Shah, J.S., W.G. Hampson, and M.I. Jayson, The distribution of surface strain in the cadaveric lumbar spine. J Bone Joint Surg Br, 1978. 60-B(2): p. 246-51.
- ↑ Krag, M.H., et al., Internal displacement distribution from in vitro loading of human thoracic and lumbar spinal motion segments: experimental results and theoretical predictions. Spine (Phila Pa 1976), 1987. 12(10): p. 1001-7.
- ↑ Schnebel, B.E., et al., A digitizing technique for the study of movement of intradiscal dye in response to flexion and extension of the lumbar spine. Spine (Phila Pa 1976), 1988. 13(3): p. 309-12.
- ↑ Fennell, A., A. Jones, and D. Hukins, Migration of the nucleus pulposus within the intervertebral disc during flexion and extension of the spine. Spine (Phila Pa 1976), 1996. 21(23): p. 2753-7.
- ↑ 18.0 18.1 Kolber, M.J. and W.J. Hanney, The dynamic disc model: a systematic review of the literature. Phys Ther Rev, 2009. 14(3): p. 181-189.