Selective Dorsal Rhizotomy in Cerebral Palsy- Selection and Physiotherapeutic Management: Difference between revisions
(Outcome measures) |
Rebecca Hall (talk | contribs) m (Selection criteria) |
||
| Line 97: | Line 97: | ||
Spasticity develops when an imbalance occurs in the excitatory and inhibitory input to α motor neurons which is caused by damage to the spinal cord and/or central nervous system. This damage then results in an imbalance between messages from the nervous system to the muscles causing increased excitability <ref>Physiopedia. (2018). ''Spasticity''. [online] Available at: <nowiki>https://www.physio-pedia.com/Spasticity</nowiki> [Accessed 26 May 2018].</ref>. | Spasticity develops when an imbalance occurs in the excitatory and inhibitory input to α motor neurons which is caused by damage to the spinal cord and/or central nervous system. This damage then results in an imbalance between messages from the nervous system to the muscles causing increased excitability <ref>Physiopedia. (2018). ''Spasticity''. [online] Available at: <nowiki>https://www.physio-pedia.com/Spasticity</nowiki> [Accessed 26 May 2018].</ref>. | ||
'''<u>Selection criteria for the SDR procedure</u>''' | '''<u>Selection criteria for the SDR procedure</u>''' | ||
Generally patients treated with SDR have bilateral lower limb spasticity and GMFCS level I – II <ref name=":5">Grunt, S., Fieggen, A. G., Vermeulen, R. J., Becher Jules, G. & Langerak Nelleke, G. (2013) Selection criteria for selective dorsal rhizotomy in children with spastic cerebral palsy: a systematic review of the literature. Developmental Medicine & Child Neurology, 56(4), 302-312.</ref>. For a patient to be considered the spasticity should have a negative impact on gross motor function and caregiver support<ref name=":5" />. 10 year follow up studies have shown that children with a GMFCS level of I – II show greater improvement than patients at level IV or V <ref name=":5" />. | |||
A paper by Nicolini-Panisson et al (2018)<ref>Nicolini-Panisson, R. D. A., Tedesco, A. P., Folle, M. R. & Donadio, M. V. F. (2018) Selective Dorsal Rhizotomy in Cerebral Palsy: Selection Criteria and Postoperative Physical Therapy Protocols. Revista Paulista de Pediatria, 36(1), 100-108.</ref> describes the use of 5 “S” as a selection criteria for patients suitable for SDR, they are as follows: | |||
Spastic: bilateral lower limb spasticity leading to reduced functionality. | |||
Strength: adequate lower limb muscle strength and control. | |||
Straight: adequate trunk and head control without fixed orthopaedic deformity. | |||
Slim: being thin. | |||
Smart: not having cognitive defects. | |||
Spasticity should be measured using the Ashworth Scale, Duncan-Ely Test, quality of deep tendon reflexes and the presence or absence of sustained clonus<ref name=":6">Robert Jones and Agnes Hunt Orthopaedic and District (2013) Criteria Used to Select Patients for Selective Dorsal Rhizotomy at RJAH, 2013. Available online: <nowiki>https://www.rjah.nhs.uk/RJAHNHS/files/d3/d383d17c-cb6f-4ebf-babd-768721503419.pdf</nowiki> [Accessed: 20.05.018]</ref>. In terms of strength it is thought that for patients to respond and be considered for SDR they should have mild weakness in their lower limbs, or the equivalent to a grade 4 on the MRC scale<ref name=":6" />. | |||
Alternatively below is a grid from Robert Jones and Agnes Hunt Orthopaedic and District <ref name=":6" />, this explains their selection criteria and is applicable to the UK hospital setting | |||
'''<u>Outcome measures</u>''' | '''<u>Outcome measures</u>''' | ||
Revision as of 10:27, 27 May 2018
Selective Dorsal Rhizotomy (SDR) is a neurosurgical procedure that aims to:[1][2]
- Reduce spasticity that interferes with motor function in children with spastic Cerebral Palsy.
- Improve function and mobility
- Increase independence
- Increase range of motion and improve positioning
Funding in the UK:
The SDR procedure is not currently available on the NHS as funding for the surgery has been withdrawn whilst NHS England examines its effectiveness via a process called Commissioning Through Evaluation. There are 7 funded centres: Alder Hey Children’s NHS Foundation Trust; The Portland Hospital for Women and Children; Great Ormond Street Hospitals NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Nottingham University Hospitals NHS Trust; University Hospitals Bristol NHS Foundation Trust; The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS[3]. They undertake approximately 120 SDR cases a year[4] with selected children to gather detailed information about clinical outcomes to produce a final report in Autumn 2018. Despite this SDR can be self-funded in the UK through the NHS hospital treatment top-up scheme[1]. In the UK the procedure costs between £30,000- £40,000[5].
Clinically relevant anatomy:
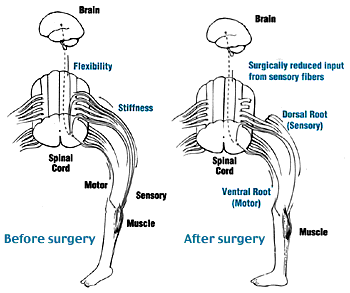
Two groups of nerve roots leave the spinal cord through the intervertebral foramen: the ventral and dorsal spinal roots [6]. The ventral nerve roots are efferent motor roots and are responsible for control of muscular contractions, hormone synthesis and gland secretion. The dorsal nerve roots are afferent sensory roots and are responsible for the transmission of sensory stimulation to the CNS [7]. The SDR procedure decreases sensory stimulation to the CNS by dividing the dorsal nerve roots whilst preserving voluntary movement [8].
Procedure:
The operation takes place under general anaesthetic and lasts approximately 5 hours[9]. The procedure involves dividing some of the lumbar sensory nerve roots in order to reduce the sensory input to the sensory–motor reflex arcs which are responsible for increased muscle tone. A laminectomy of one or more vertebrae from L1 to S1 vertebrae is performed to expose the dural sac [10], which is opened to display the spinal conus with or without the cauda equina. The sensory nerve roots are identified intraoperatively using electrical stimulation, those that generate unusual electrical activity are thought to be those which contribute to spasticity. The selected sensory rootlets are divided, preserving some sensory supply and the motor roots responsible for voluntary movements[11]. Up to 50% of the sensory nerve at each level is divided[12]. SDR is common in North America however there are significant variations between centers in the way the procedure is carried out[13].
For a descriptive video of the procedure follow this link to Dr. Samuel Brown discussing Selective Dorsal Rhizotomy [14].
https://www.youtube.com/watch?v=HFad8MiTK_g
Advantages and Disadvantages of the different surgical approaches for SDR [13].
| Procedure | Advantages | Disadvantages |
| Multi-level laminectomy L1-S1 | The root level can be easily determined,
dorsal root can be usually be separated readily from the ventral root at each level the amount of each dorsal root cut can be easily tailored for the individual clinical situation the spinal cord is not at risk of damage the procedure is readily and safely accomplished with loupes or no magnification |
Long surgical incision
Extensive muscular dissection the laminae are cut at multiple levels, the ventral roots may be damaged during separation from the dorsal roots significant postoperative pain. |
| Single or Bi-level laminectomy at the level of the conus | Small surgical incision
Small amount of muscle dissection Fewer lamine are cut Less postoperative pain Avoidance of the ventral roots |
More technically demanding procedure and magnification with an operating microscope is required for safety
More difficult to determine root levels More difficult to tailor the operation to the individual clinical situation Conus at risk |
SDR has been shown to be a safe and effective to reduce spasticity of children in all Gross Motor Function Classification System Levels (GMFCS) with the benefits lasting into adolescence and adulthood[2][16][17].
Associated risks:
SDR is a high-risk surgery and the procedure is irreversible. Patients may experience deterioration in walking ability, numbness and bladder dysfunction following the operation and later complications including spinal deformity and hip dislocation[11][12]. Scoliosis is generally associated with the traditional multi-level laminectomy technique which exposes about 3 inches of the lower spine[12]. Other suggested adverse events include death, worsening motor function and/or paraplegia, infection of the surgical wound, meningitis, cerebrospinal fluid leakage, constipation, weakness, chronic pain, and late arachnoiditis and/or syringomyelia [11].
Cerebral Palsy (CP)
CP is a condition that encompasses brain disorders that originate in early childhood or during foetal stages of development. Abnormalities in balance, posture, movement, language and vision are common. These variants can lead to a reduced level of function in activities such as walking and sitting, often with increased levels of discomfort[11].
Current conservative treatments in CP include:
- Oral muscle relaxant medication
- Orthotic devices
- Physiotherapy
- Repeated intramuscular injection of botulinum toxin
Surgical interventions include:
- Tendonotomy
- Tendon lengthening
- Peripheral neurotomy
- Osteotomy
- Electrical stimulation of muscles or dorsal spinal cord
- Continuous intrathecal baclofen infusion [11]
80% of CP patients experience lower limb spasticity[11].
“Spasticity is a motor disorder characterised by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neurone syndrome.”[18]
Spasticity develops when an imbalance occurs in the excitatory and inhibitory input to α motor neurons which is caused by damage to the spinal cord and/or central nervous system. This damage then results in an imbalance between messages from the nervous system to the muscles causing increased excitability [19].
Selection criteria for the SDR procedure
Generally patients treated with SDR have bilateral lower limb spasticity and GMFCS level I – II [20]. For a patient to be considered the spasticity should have a negative impact on gross motor function and caregiver support[20]. 10 year follow up studies have shown that children with a GMFCS level of I – II show greater improvement than patients at level IV or V [20].
A paper by Nicolini-Panisson et al (2018)[21] describes the use of 5 “S” as a selection criteria for patients suitable for SDR, they are as follows:
Spastic: bilateral lower limb spasticity leading to reduced functionality.
Strength: adequate lower limb muscle strength and control.
Straight: adequate trunk and head control without fixed orthopaedic deformity.
Slim: being thin.
Smart: not having cognitive defects.
Spasticity should be measured using the Ashworth Scale, Duncan-Ely Test, quality of deep tendon reflexes and the presence or absence of sustained clonus[22]. In terms of strength it is thought that for patients to respond and be considered for SDR they should have mild weakness in their lower limbs, or the equivalent to a grade 4 on the MRC scale[22].
Alternatively below is a grid from Robert Jones and Agnes Hunt Orthopaedic and District [22], this explains their selection criteria and is applicable to the UK hospital setting
Outcome measures
The NICE guidelines suggest that outcome measures should include:
- the incidence of neurological impairment and spinal deformity;
- the need for additional operations;
- and assessments of disability, social inclusion, and quality of life.[23]
Previously, long-term functional outcomes, safety and side effects have been measured 5 years post SDR. The Gross Motor Function Classification System (GMFCS) levels III–V wree used to quantify these outcomes. It found that there was and reduce in muscle tone of the hip abductors, hamstrings and dorsiflexors with increased PROM in hip abduction, popliteal angle and ankle dorsiflexion. This showed that SDR provides lasting functional benefits over a period of at least five years postoperatively[24].
Medical management following the SDR procedure
Physiotherapeutic management following the SDR procedure
Clinical bottom line
References
- ↑ 1.0 1.1 Verity, C. (2017). Selective Dorsal Rhizotomy (SDR) | Disability charity Scope UK. [online] Available at: https://www.scope.org.uk/support/therapies/selective-dorsal-rhizotomy [Accessed 20 May 2018].
- ↑ 2.0 2.1 Nordmark, E., Josenby, A., Lagergren, J., Andersson, G., Strömblad, L. and Westbom, L. (2008). Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatrics, 8(1).
- ↑ Scope UK. (2018). Selective Dorsal Rhizotomy (SDR) | Disability charity Scope UK. [online] Available at: https://www.scope.org.uk/support/therapies/selective-dorsal-rhizotomy [Accessed 22 May 2018].
- ↑ NHS England. (2014). NHS England » NHS England funds the evaluation of specialist surgery for more than 100 children a year with cerebral palsy. [online] England.nhs.uk. Available at: https://www.england.nhs.uk/2014/07/cte-specialist-surgery/ [Accessed 20 May 2018].
- ↑ The Portland Hospital (2015). The Portland Hospital – the first private hospital in the UK to perform the Selective Dorsal Rhizotomy procedure | News | The Portland Hospital. [online] Available at: http://www.theportlandhospital.com/news/the-portland-hospital-the-first-private-hospital-in-the-uk-to-perform-the-selective-dorsal-rhizotomy-procedure/ [Accessed 22 May 2018].
- ↑ St. Louis Children's Hospital (2018). Selective Dorsal Rhizotomy. [image] Available at: http://www.stlouischildrens.org/our-services/center-cerebral-palsy-spasticity/about-selective-dorsal-rhizotomy-sdr [Accessed 21 May 2018].
- ↑ Laser Spine Institute (n.d.). Guide to a Ventral Nerve Root. [online] Laser Spine Institute. Available at: https://www.laserspineinstitute.com/back_problems/spinal_anatomy/spinal_cord/ventral_root/ [Accessed 21 May 2018].
- ↑ NHS England. (2013). Clinical Commissioning Policy Statement : Selective Dorsal Rhizotomy (SDR) [online] Available at: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2013/04/e09-ps-a.pdf [Accessed 21 May 2018].
- ↑ NICE (2006). Interventional procedure overview of selective dorsal rhizotomy for spasticity in cerebral palsy | NICE. [online]Available at: https://www.nice.org.uk/guidance/ipg373/evidence/overview-pdf-316141021 [Accessed 20 May 2018].
- ↑ Funk, J. and Haberl, H. (2016). Monosegmental laminoplasty for selective dorsal rhizotomy—operative technique and influence on the development of scoliosis in ambulatory children with cerebral palsy. Child's Nervous System, 32(5), pp.819-825.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 NICE (2010). Selective dorsal rhizotomy for spasticity in cerebral palsy | Guidance and guidelines | NICE. [online] Available at: https://www.nice.org.uk/guidance/ipg373/chapter/2-The-procedure#outline-of-the-procedure [Accessed 17 May 2018].
- ↑ 12.0 12.1 12.2 The Robert Jones and Agnes Hunt Orthopaedic Hospital. (2014). RJAH Hospital Film. [online] Available at: https://www.rjah.nhs.uk/Our-Services/ORLAU/Selective-Dorsal-Rhizotomy.aspx [Accessed 20 May 2018]
- ↑ 13.0 13.1 Steinbok, P. (2007). Selective dorsal rhizotomy for spastic cerebral palsy: a review. Child's Nervous System, 23(9), pp.981-990.
- ↑ Seattle Children's SDR channel (2017). Dr. Samuel Browd discusses Selective Dorsal Rhizotomy (SDR). [video] Available at: https://www.youtube.com/watch?v=HFad8MiTK_g [Accessed 21 May 2018].
- ↑ St. Louis Children's Hospital (2018). Selective Dorsal Rhizotomy. [image] Available at: http://www.stlouischildrens.org/our-services/center-cerebral-palsy-spasticity/about-selective-dorsal-rhizotomy-sdr [Accessed 21 May 2018].
- ↑ Dudley, R., Parolin, M., Gagnon, B., Saluja, R., Yap, R., Montpetit, K., Ruck, J., Poulin, C., Cantin, M., Benaroch, T. and Farmer, J. (2013). Long-term functional benefits of selective dorsal rhizotomy for spastic cerebral palsy. Journal of Neurosurgery: Pediatrics, 12(2), pp.142-150.
- ↑ Ailon, T., Beauchamp, R., Miller, S., Mortenson, P., Kerr, J., Hengel, A. and Steinbok, P. (2015). Long-term outcome after selective dorsal rhizotomy in children with spastic cerebral palsy. Child's Nervous System, 31(3), pp.415-423.
- ↑ Lance JW. Symposium synopsis. In: Feldman RG,fckLRYoung RR, Koella WP (eds). Spasticity: Disordered Motor Control. Chicago, IL: Year Book 1980:485–94.
- ↑ Physiopedia. (2018). Spasticity. [online] Available at: https://www.physio-pedia.com/Spasticity [Accessed 26 May 2018].
- ↑ 20.0 20.1 20.2 Grunt, S., Fieggen, A. G., Vermeulen, R. J., Becher Jules, G. & Langerak Nelleke, G. (2013) Selection criteria for selective dorsal rhizotomy in children with spastic cerebral palsy: a systematic review of the literature. Developmental Medicine & Child Neurology, 56(4), 302-312.
- ↑ Nicolini-Panisson, R. D. A., Tedesco, A. P., Folle, M. R. & Donadio, M. V. F. (2018) Selective Dorsal Rhizotomy in Cerebral Palsy: Selection Criteria and Postoperative Physical Therapy Protocols. Revista Paulista de Pediatria, 36(1), 100-108.
- ↑ 22.0 22.1 22.2 Robert Jones and Agnes Hunt Orthopaedic and District (2013) Criteria Used to Select Patients for Selective Dorsal Rhizotomy at RJAH, 2013. Available online: https://www.rjah.nhs.uk/RJAHNHS/files/d3/d383d17c-cb6f-4ebf-babd-768721503419.pdf [Accessed: 20.05.018]
- ↑ NICE (2018). Selective dorsal rhizotomy for spasticity in cerebral palsy | Guidance and guidelines | NICE. [online] Nice.org.uk. Available at: https://www.nice.org.uk/guidance/ipg373/chapter/1-Guidance [Accessed 26 May 2018].
- ↑ Nordmark, E., Josenby, A., Lagergren, J., Andersson, G., Strömblad, L. and Westbom, L. (2008). Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatrics, 8(1).
Editing in process.
26/05/18