PT 858: Charcot-Marie-Tooth Disease Case Study
Original Editor -
Top Contributors -
Abstract[edit | edit source]
This is a fictional case study of a 25-year-old male diagnosed with subtype 1A Charcot-Marie-Tooth disease (CMT). CMT is a hereditary group of disorders characterized by sensory loss, muscle atrophy, and weakness.[1] This case study documents the initial patient assessment with a new physiotherapist one month after the patient moved to a new city. It includes the subjective and objective findings, as well as patient-centered goals to work toward achieving in subsequent therapy sessions. The patient’s primary complaints were more noticeable symptoms progressing in the past four months contributing to tripping when running and numerous sprained ankles. Based on these complaints, patient-centered goals were developed, and evidence-based interventions were implemented to help the patient meet these goals and maintain participation in meaningful activities. The patient will come for treatment weekly for four weeks and all outcome measures will be reassessed at this time. Future appointments will likely be more spread out following this depending on patient progress.
Introduction[edit | edit source]
This case study will examine a 25-year-old male diagnosed with subtype 1A Charcot-Marie-Tooth disease (CMT), also known as hereditary motor sensory neuropathy (HMSN). [1][2] CMT is the name given to a group of disorders characterized by sensory loss, muscle atrophy, and weakness due to peripheral nerve deterioration. [1] It is one of the most common hereditary conditions and can be inherited in one of three ways: autosomal dominant, autosomal recessive, or X-linked. In autosomal recessive CMT, the child would receive two mutated genes, one from each parent. Neither parent would have the disease, but they would be a carrier. In autosomal dominant CMT, the child would have a 50% chance of inheriting the disease from a carrier parent, and would only need to receive one copy of the mutated gene. In X-linked CMT, the mutation is associated with the “X” sex chromosome. Females have two “X” chromosomes, one from each parent, and males only have one, from their mother. This means that in X-linked CMT, a male child would have a 50% chance of receiving the mutated “X” chromosome from his mother. [3] A female child has the same chance of receiving the mutated “X” chromosome from their mother, however, they are likely to be less affected due to the random inactivation hypothesis proposed by Mary Lyon in 1961.[4]
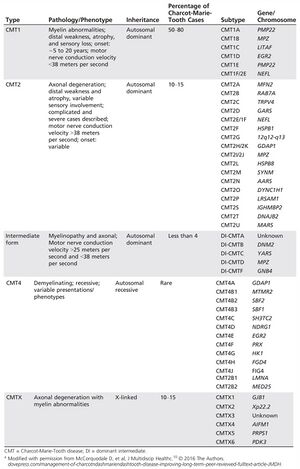
The most common form of CMT is type 1 (CMT1), accounting for over 50% of cases. Of this, subtype 1A is the most prevalent. This is due to an extra copy or duplication of the peripheral myelin protein 22 (PMP22) gene.[6] Scientists still don’t understand the exact implication of this extra copy; however, it is known that it alters the myelin sheaths of Schwann cells. There are other different types (CMT2, CMT4, CMTX and intermediate form) and subtypes, which can be seen in Figure 1.[5]
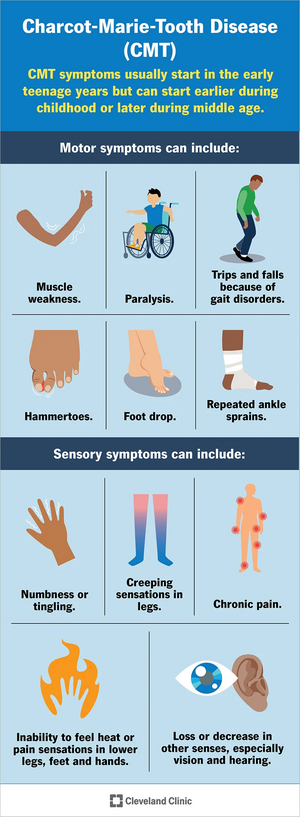
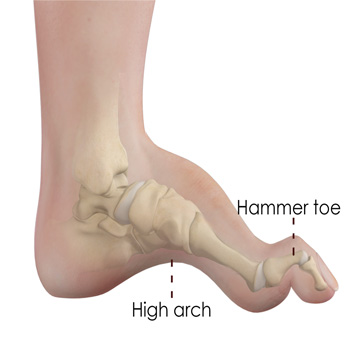
Symptoms of CMT1A vary among individuals, and onset occurs before 20 years old. The progression of the disease is gradual, and the feet are commonly affected first.[8] Individuals noticed weakness, atrophy, and increased fatigue in the feet and legs.[9] Therefore, it is common to see abnormalities develop like pes cavus, drop foot, and/or hammer toes.[10] This can contribute to balance problems and increase the patient’s risks of falling. As the disease progresses, similar weakness and atrophy is seen in the hands and arms, leading to decreased dexterity. Additionally, it is common for individuals with CMT to experience sensory problems, such as decreased proprioception (position sense) and kinesthesia (movement sense). It is also common for patients to report pain, paresthesia, or reduced sensations in the extremities.[7] As the disease progresses, there can be decreased sensation in hearing or vision.[3] In severe cases, it may influence the diaphragm and respiration.[8]
The purpose of this case study is to provide readers with an example of how an individual may present with CMT and how physiotherapy can be used to help manage the condition. It is important to note that symptoms and severity can vary among individuals and interventions that work for one patient may not be successful for another patient[2][3][11] however, this case study can provide a free and easily accessible reference to clinicians regarding patient symptoms and possible treatment options.
Client Characteristics[edit | edit source]
The patient is a 25-year-old male. He works as an accountant and recently moved to the city for a job. He was diagnosed with autosomal dominant Charcot-Marie-Tooth (CMT) disease subtype 1A (CMT1A) by a neurologist at the at of 14. To manage his symptoms, he typically sees a physiotherapist once a month, however, due to the busyness of the move and his schedule, he was unable to see his physiotherapist for the 3 months leading up to moving and was unable to get an appointment with a new physiotherapist until now, one month after he moved. The patient has had multiple ankle sprains in the past 4 months, and in the past month has been unable to run due to an increased frequency of tripping over his own feet when increasing speed, in addition to general pain on the plantar surface of the foot. He reports feeling frustrated that he hasn’t be able to run and having a decreased mood. The patient self-referred to physiotherapy for maintenance of his condition and treatment.
Examination Findings[edit | edit source]
Subjective[edit | edit source]
History of Present Illness
In the past month, the patient has noticed that anytime he tried to increase his pace while walking, he found himself tripping over his feet and had to slow down to avoid falling. Prior to this, the patient reports tripping over his feet while running, and he believes this to be the cause of his recent sprained ankles. He reports that prior to 4 months ago, he would have ankle sprains occasionally, around once every year. However, in the past 4 months, he reports having one ankle sprain roughly every six weeks, with both ankles having been affected. The patient has not yet had any treatment for his ankle sprains, due to his missed appointments, but has received physiotherapy for treatment of CMT prior.
With respect to patient pain, he has reported an increase in plantar foot pain bilaterally in the past 3 months. The patient reports an increase in pain with walking over the last month. In addition, he also reports an increase in fatigue throughout the day, finding it more challenging to complete tasks around his home, such as cooking and cleaning.
Past Medical History
The patient has had previous ankle sprains to both his left ankle (7 and 5 years ago) and his right ankle (4 and 3 years ago). He went to physio for the first ankle sprain 7 years ago, but not for the others.
In the past 4 months, he has had one sprain to his left ankle and 2 to his right ankle.
A screening test for major depression disorder indicated a possible co-morbidity. This screen was conducted because depression is common in CMT patients. CMT is not a curable disease, so it is important to think about factors associated with quality of life (QoL) that are amenable to treatment[12]. As physiotherapists we should be screening for yellow and orange flags; this will provide valuable knowledge for treatment/disease management. This is applicable for CMT since there are higher rates of depression, anxiety and general distress compared to controls in studies [13] [12][14]. Other health conditions that can be seen in CMT1A and may be considered when assessing this patient include fatigue[12], constipation, hearing loss, erectile dysfunction, urinary incontinence[14].
Medications
Ibuprofen (400 mg) 2 times per day for pain in ankles.
Fluoxetine (30 mg) once per day in the morning, though the patient reports that he often forgets to take it.
Health Habits
No history of smoking and consumes 2-3 alcoholic beverages per week.
Family History
His mother had autosomal dominant CMT type 1A, diagnosed when she was 16 years old.
Social History
The patient works as an accountant and lives alone in a one-bedroom apartment on the second floor of a building in a mid-sized city. There are 5 flights of stairs total inside the building, but there is an elevator. The patient’s parents and siblings live in another city around 3 hours away. Prior to his move, the patient frequently participated in a weekly run club. He has found a new run club to join since moving but has yet to join due to his current condition.
Functional History
The patient was independent in iADLs and bADLs. He did not use a gait aid, but has rearfoot varus wedge accommodative orthotics for his pes cavus. Prior to his ankle sprains, was running an average of 10-12 km per week and strength training 3 times each week .
Current Functional Status
He does not currently use gait aids or adaptive equipment, but is still wearing his orthotics. The patient feels he is moving slower and movements are more effortful, as well as that his sense of balance is not the same.
At home, he chooses to take the elevator instead of walking the 2 flights of stairs to his third floor apartment and in recent weeks, has found he is too tired to cook, which he enjoys doing, and orders take-out more than usual.
Objective[edit | edit source]
Observation
- Mild (R) mid-thoracic scoliosis
- UE muscle tone appears normal
- Bilateral pes cavus, more extreme arch of (R) foot, varus heel angle from posterior POV
- Bilateral hammer toes
- Distal mild muscle wasting of bilateral lower legs, stork inverted champagne bottle appearance notable on anterio-lateral leg
Range of Motion
Upper Extremity - All joints within normal limits for passive and active range
Lower Extremity - Hip extension: 14 degrees (R), 16 degrees (L); Ankle Dorsiflexion: 5 degrees (R), 6 degrees (L)
***all end feels normal
Manual Muscle Testing
| Grade /5 (R) | Grade /5 (L) | |
| Hip Extension | 4+ | 4+ |
| Hip Flexion | 4 | 4 |
| Knee Extension | 5 | 5 |
| Knee Flexion | 4+ | 5 |
| Ankle Dorsiflexion | 3 | 3+ |
| Ankle Plantar Flexion | 4 | 4 |
| Ankle Inversion | 3+ | 3+ |
| Ankle Eversion | 5 | 5 |
Specific Muscle Strength Testing: determined tibialis anterior, extensor digitorum longus and extensor hallicus longus are primarily responsible for decreased strength of foot dorsiflexors and inverters
Neurological Assessment
Dermatomes: no deficits above the knee bilaterally. Below the knee does not follow a specific dermatomal pathway - patient presenting with altered sensation bilaterally following a stocking pattern running from distal ankle to toes.
UMN: Babinski (-), Hoffmann’s (-), Oppenheimer’s (-)
LMN/Deep Tendon Reflexes:
- C6: Biceps Tendon– 2 (normal)
- C7: Triceps Tendon– 2 (normal)
- L3L4: Patellar Tendon – 2 (normal)
- S1S2: Achilles Tendon – 1 (hyporeflexia)
Special Tests: Upper Extremity - Spurlings (-), Distraction (-), Vertebral Artery Test (-); Lower Extremity - Slump (-), Straight Leg Raise (-)
Sensation Testing:
A) Superficial Sensations: Light touch, temperature sense, and sharp/dull discrimination intact above the knee and impaired below the knee bilaterally.
B) Deep Sensations: Vibration sense intact above the knee and impaired below the knee bilaterally. Proprioception/Kinesthesia impaired bilaterally for ankle dorsiflexion/plantar flexion.
Balance:
- Romberg Test (+) - loss of balance within 15 seconds with eyes open and 5 seconds with eyes closed
- Mini Best Score: 22/28
Observational Gait Analysis
Decreased stride length and increased time in double support were noted with no observable differences in stance phase, swing phase, or arm swing. There were noticeable bilateral pes cavus, distal mild bilateral lower legs, and anterior pelvic tilt. Increased compensation was observable at bilateral hips (hip hiking and circumduction). Bilateral mild foot drop was also observed. Using the 10m walk test, a gait speed of 1.2m/sec was calculated, which is lower than age normative values. Patient completed repeated heel walking for 20m, during the last 10m of the task bilateral reduction in ankle dorsiflexion was noted.
Outcome Measures
| Outcome measure | Score | Clinical impression |
|---|---|---|
| CMTES-R | 11 | Moderate classification of disease status[15] |
| Mini-BEST | 22/28 | Indicates balance deficits. The cut-off score in other neurological population such as Parkinson's is 21/28 to distinguish those with vs without balance deficits[16]. |
| Modified fatigue impact scale (MFIS) | 38/84 | Score of >38 indicates abnormal fatigue[17]. |
| Functional Gait Assessment (FGA) | 25/30 | The average score for different neurological conditions (stroke, Parkinson's, vestibular disorders) tends to be around 20/30 [18][19] |
Clinical Impression[edit | edit source]
Analysis[edit | edit source]
Patient is a 25-year-old male with worsening lower extremity instability over the last 4 weeks that has impeded his ability to run. He presents with impaired balance, proprioception, reduced lower extremity strength, and overall reduced physical activity. Patient is a good candidate for physical therapy activities aimed at increasing strength of lower extremities, increasing dynamic balance, education on pacing, and optimizing participation in previously enjoyed physical activities.
Prognosis[edit | edit source]
Although the patient has CMT type 1A that will continue to progress, their previous high level of function plus activity, and young age are positive indicators for slowed progression of disability associated with his condition.
Problem List[edit | edit source]
-Impaired balance and sensation in bilateral lower extremities below the knee
-Reduced range of motion in bilateral dorsiflexion and hip extension
-Reduced strength of bilateral ankle dorsiflexors and inverters
-Decreased gait speed
-Unable to participate in run club
Intervention[edit | edit source]
Patient-centered Treatment Goals[edit | edit source]
1. Impaired Balance and Sensation in LE Below the Knee
Short Term Goal By 3 weeks the patient will have increased his mini-BEST score by 2 points using postural balance training and core stability exercises, and therefore classifying his score as lack of balance deficits. The patient may have up to 3 sensory modifications made as per intervention guidelines.
Long Term Goal After 2 months of his balance and core stability exercise program, the patient’s mini-BEST score will have increased to 24/28 with minimal (1-2) changes in sensory information required compared to initial assessment.
2. Reduced Dorsiflexion and Hip Extension ROM
Short Term Goal By the beginning of the patient’s third week in treatment, his dorsiflexion will have increased by at least 4 degrees and hip extension by 3 degrees bilaterally after mobilizations, stretching and use of AFO.
Long Term Goal After 6 weeks of treatment including mobilizations, stretching and increased duration of AFO use, the patient will have increased dorsiflexion by an additional 6 degrees and hip extension by at least 4 degrees bilaterally.
3. Reduced Strength of Ankle DF and Inverters
Short Term Goal After 3 weeks of treatment, the patients dorsiflexors and invertors strength will have not declined, noting that the progression of his weakness has not persisted. Dorsiflexors in the right lower leg will remain at a 3/5, left dorsiflexors at a 3+/5, while invertors will be maintained at a 3+/5 bilaterally.
Long Term Goal After 3 months of his strengthening program, the patient will have progressed to completing his exercises at 60% 1RM 3 times per week without reducing this intensity during his sessions. At this time, he will also not have any loss in his dorsiflexor or invertor MMT strength grading thus showing no progression of muscular weakness.
4. Unable to Participate in Run Club
Short Term Goal In two weeks patient will be able to walk 20 m 5 times with breaks in between, while using a safe heel walking technique without reporting higher than a 4 on the RPE scale or any other symptom or deficit requiring him to stop.
Long Term Goal In 2 months, the patient will have attended 90% of the scheduled walks with the group, and is able to participate in walking with proper heel walking technique in 10 minute bouts below an RPE of 4, and with no other symptom or deficit requiring him to stop.
5. Decreased Gait Speed
Short Term Goal By the start of the sixth week in the walk program the patient will see an increase in gait speed during a 10 m walk test to a typical value for his age (about 1.35 m/s) , as he will also be participating in the neuromuscular disease walk club, balance training, as well as strengthening of the dorsiflexors and invertors.
Long Term Goal After 6 months of treatment for balance, strength, gait speed, and gait technique, the patient will be able to continue the walk run program by completing at least 10 minutes of his 30 minute total by jogging at a low intensity with minimal difficulty.
Management Program[edit | edit source]
Technology[edit | edit source]
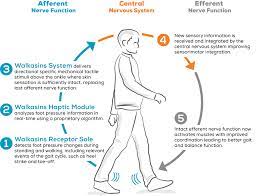
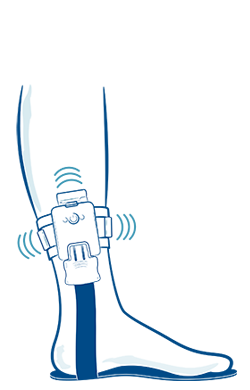
Due to the motor and sensory neuropathy, balance and gait are common impairments in individuals with CMT.[22] Ankle foot orthoses (AFOs) are commonly prescribed to individuals with CMT to compensate for the progressive limb weakness and sensory deficits that cause these impairments.[22] An emerging technology for the management of sensory peripheral neuropathy is a wearable sensory prosthesis for the foot and ankle.[23] Specifically, a device called the walkasins© demonstrates results in improving functional gait, balance, and decreasing falls risk factors and falls rate in individuals with sensory peripheral neuropathy.[24][25] This device works by replacing the lost sensation in the plantar aspect of the foot through information gathered in the sole of the device about foot placement and provides this information through mechanical stimuli of a unit higher up the limb where sensation is better intact.[25] This afferent feedback occurs in real time and provides the user with the necessary sensory input for safe and effective ambulation.[23] Over time, this device may produce neural modulatory changes in the brain related to gait and balance tasks because of this supplementary stimulation.[26] In addition to resistance training, balance, and gait training with physiotherapy, this device may provide our patient with a more functional gait and a decreased falls risk.
Although not yet specifically trialed in the CMT population, sensory neuropathy is a significant presentation in this condition, and there is an opportunity for it to be used in CMT management. In this case, the device may better give our patient the ability to safely run again with the proprioceptive and touch sensation the device provides, than physiotherapy training alone.
However, there are challenges presented with the device regarding its accessibility. The device is not yet widely available to consumers, however healthcare providers, including PT’s, can fill out a form on the company website that may allow access to the device. In addition to the physical barriers of accessing the device, a financial barrier is presented as the device costs $4,400 and is not covered by insurance.[27] Finally, it is recognized that although this could provide great benefit for the patient currently, CMT is a progressive condition and as it worsens and the lower limb is affected more proximally, the device may no longer be feasible for this patient.
Outcome[edit | edit source]
Four main outcome measures were used to examine areas of ICF and provide baselines of how CMT is impacting this patient.

The first outcome measure was the Rash analysis based Charcot Marie Tooth Examination Score (CMTES-R). The CMTES-R scale is more sensitive than the original CMTES and demonstrates more change over time in patients with CMT1A. The CMTES-R is most sensitive to change in patients with mild to moderate baseline disease severity[29], aligning with this patients case. When looking at the ICF this outcome measure can help distinguish the overall health condition and monitor progression of the severity. Monitoring the severity of disease can be a good indicator of whether treatment is working or when treatment/management focus needs to shift; for example changing the focus of physiotherapy sessions from remediation to compensation. When the Rash analysis can be feasibly implemented it is a good outcome measure to use with patients. If not, practitioners can use the CMTES without rash analysis.
Historically the most used outcome measure in CMT1A to quantify clinical severity and measure disease progression has been the Charcot Marie tooth neuropathy score (CMTNS). To minimize floor and ceiling effects the CMTNS was revised yielding the CMTNS version 2 (CMTNSv2). To address clustering of disease stages the Rasch-modified CMTNS (CMTNS-R) scores were developed. In place of the CMTNS-R the CMTES-R is used because it does not include repeated nerve conduction evaluation and it is implemented in clinics.
The second outcome measure was the mini-BEST scale. The mini-BEST is appropriate to assess body function and activity domains of the ICF. The mini-BEST scale appears to have strong test psychometrics across neurological populations with good clinical utility[22]. To assess the impact CMT has on this patient in terms of body structure and subsequent activity the mini-BEST will assess postural control and specific systems affected. It is one of the few balance assessments that includes response to loss of balance which would be appropriate for our patient's moderate disease severity and young age. The mini-BEST has high content validity since individual items are part of well-known balance batteries such as Berg Balance scale, Clinical Test of Sensory Integration in Balance, Dynamic Gait Index and TUG[22].
The third outcome measure utilized was MFIS. MFIS is a commonly used questionnaire to assess perceived fatigue and has been validated in many neurological disorders[23]. The MFIS can evaluate body structure/function, activity, and participation domains of the ICF [30]. The MFIS has physical, cognitive and psychosocial sub scores which can give insight into possible struggles and education needed for this patient. The psychosocial component of the scale may be of interest since this patient has a diagnosis of major depression disorder; there are two psychosocial questions that ask about motivation to participate in social activities and ability to do things away from home. The questions are scored from 0-4 and a higher score overall indicates greater impact of fatigue on a person's activities, this patient scored 38/84 indicating that fatigue is limiting activities.
The final outcome measure incorporated was the FGA. The FGA will analyze both activity and participation within the ICF domain for this patient [31]. Walking is an important activity within the ICF. This patient also enjoys running so a functional walk test will indicate the likelihood of returning to his running club, allowing us to consider the participation domain of the ICF. The FGA is a modified version of the functional gait index to improve reliability and decrease the ceiling effect. This scale would provide more of a challenge for our patient since it looks at gait with a narrow base of support, ambulating backwards and gait with eyes closed. The FGA also provides more external validity than tests like TUG or 6-minute walk test (typically done in a flat hallway with no distractions) since it has variability and challenging skills[25]. The highest score is 30/30 indicating normal ambulation. The average score for different neurological conditions (Stroke, Parkinson's, vestibular disorders) tends to be around 20/30[25] [12].
Discussion[edit | edit source]
The patient was a 25-year-old male who self-referred to physiotherapy for management and treatment of his CMT. An initial assessment was conducted and revealed some expected observation findings for CMT such as bilateral pes cavus, mild muscle wasting of bilateral lower legs, and bilateral hammer toes. Range of motion suggests a slight decrease in right dorsiflexion (5 degrees on the right compared to 6 degrees on the left) and hip extension (14 degrees on the right compared to 16 degrees on the left), and bilateral weakness in ankle inversion (3+/5 bilaterally) and dorsiflexion (3/5 on the right and 3+/5 on the left). The patient also had impaired superficial and deep sensations below the knee, with light touch, temperature sense, and sharp/dull discrimination impaired bilaterally, as well as vibration sense impaired bilaterally, and proprioception/kinesthesia impaired bilaterally for ankle dorsiflexion/plantar flexion. This was also shown through the Romberg test, as the patient lost their balance within 15 seconds with eyes open and 5 seconds with eyes closed. Hip compensation and decreased gait speed were the main observations during gait analysis. A gait speed of 1.2 m/s was calculated using the 10 m walk test, which is a slower gait speed than expected for a patient of this age. The patient also reported bilateral pain on the plantar aspect of the foot that has increased within the past three months, in addition to having multiple ankle sprains in the past 4 months and being unable to run due to an increased frequency of tripping over his own feet when increasing in speed.
The outcome measures assessed with the patient were the CMTES-R, Mini-BEST, MFIS, and FGA. On the CMTES-R, the patient scored 11, which correlates to a moderate classification of disease status.[15] With the Mini-BEST, the patient scored 22/28 which indicates balance deficits.[16] On the MFIS, the patient scored 38/84. This is right at the cut-off point for indicating abnormal fatigue, which is any score greater than 38.[17] Lastly, on the FGA, the score was 25/30.
Based on these findings, reduced strength of ankle dorsiflexors and inverters, decreased gait speed, and an inability to participate in run club were the main physiotherapy goals identified. These patient-centered goals were developed to help the patient decrease the disability associated with this disease, and to help him maintain participation in his meaningful activities. To address these goals, various interventions will be implemented. To address the strength deficits in the ankle dorsiflexors and inverters, the patient will be prescribed a 12-week home resistance-based, progressive exercise protocol. To increase the patient’s gait speed, he will begin a walk-run program. Furthermore, while the patient works on increasing his gait speed and getting back to running, he will be offered the chance to participate in a group exercise club tailed towards individuals with CMT and other neuromuscular diseases that consists of biking, walking or running as a group for 30 minutes 3 times per week for 12 weeks. Moreover, an emerging technology was presented as an intervention option that could help to improve the patient’s functional gait and balance, as well as decrease falls risk factors and falls rate. In addition to his physiotherapy treatment plan, the patient will also be referred to an occupational therapist for assistance with his lack of social interaction/participation, a psychologist to address his psychosocial concerns, and a podiatrist for further assistance with his CMT symptoms related to his feet, such as the bilateral pes cavus and hammer toes.
In conclusion, this fictional case study presented a 25-year-old male diagnosed with CMT. An initial assessment complete with a subjective history and objective examination was presented, which were used to guide the development of a treatment plan to address patient-centered goals. A novel technology was also identified as a potential treatment option for the patient. This case study aimed to demonstrate what a potential initial assessment and treatment plan could look like for a patient with a CMT diagnosis, as well as to provide a resource for clinicians to reference when working with not just CMT patients, but other neurological populations as well.
Self-Study Questions[edit | edit source]
1. Which of the following is not a type of Charcot-Marie-Tooth disease?
a) CMT1
b) CMT2
c) CMT3
d) CMT4
e) CMTX
Answer [c]
2. Which of the following is a symptom that a person with CMT may present with?
a) Muscle hypertrophy
b) Hyper-reflexia
c) Hypertonia
d) Loss or decrease in other senses
e) All of the above
f) None of the above
Answer [d]
3. The following are common conditions co-occurring with Charcot-Marie-Tooth disease:
a) Depression
b) Chronic heart failure
c) Fatigue
d) Gout
e) A and C
Answer [e]
Additional Patient Resources[edit | edit source]
Muscular Dystrophy Association - Charcot-Marie-Tooth Disease
Charcot-Marie-Tooth Association
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Charcot-Marie-Tooth Disease (CMT) [Internet]. Muscular Dystrophy Association; 2021 [cited 2023 May 8]. Available from: https://www.mda.org/disease/charcot-marie-tooth
- ↑ 2.0 2.1 Reilly MM, Murphy SM, Laurá M. Charcot-Marie-Tooth disease. Journal of the Peripheral Nervous System. 2011 Apr 19;16(1):1–14. doi:10.1111/j.1529-8027.2011.00324.x
- ↑ 3.0 3.1 3.2 Charcot-Marie-Tooth Disease [Internet]. U.S. Department of Health and Human Services; 2023 [cited 2023 May 8]. Available from: https://www.ninds.nih.gov/health-information/disorders/charcot-marie-tooth-disease
- ↑ Lyon MF. Gene action in the X-chromosome of the mouse (mus musculus L.). Nature. 1961 Apr 1961;190(4773):372–3. doi:10.1038/190372a0
- ↑ 5.0 5.1 Ramchandren S. Charcot-Marie-Tooth disease and other genetic polyneuropathies. CONTINUUM: Lifelong Learning in Neurology. 2017 Oct;23(5):1360–77. doi:10.1212/con.0000000000000529
- ↑ Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clinical Genetics. 1974 Aug;6(2):98–118. doi:10.1111/j.1399-0004.1974.tb00638.x
- ↑ 7.0 7.1 Charcot-Marie-Tooth disease (CMT): Symptoms & treatment [Internet]. Cleveland Clinic; 2022 [cited 2023 May 8]. Available from: https://my.clevelandclinic.org/health/articles/6009-charcot-marie-tooth-disease-cmt
- ↑ 8.0 8.1 Charcot-Marie-Tooth Disease Type 1A [Internet]. U.S. Department of Health and Human Services; 2023 [cited 2023 May 8]. Available from: https://rarediseases.info.nih.gov/diseases/1245/charcot-marie-tooth-disease-type-1a
- ↑ Marques W, Freitas MR, Nascimento OJ, Oliveira AB, Calia L, Melo A, et al. 17P duplicated Charcot–Marie–Tooth 1a. Journal of Neurology. 2005 Mar 18;252(8):972–9. doi:10.1007/s00415-005-0797-9
- ↑ Bienfait HME, Verhamme C, Schaik IN, Koelman JHTM, Visser BW, Haan RJ, et al. Comparison of CMT1A and CMT2: Similarities and differences. Journal of Neurology. 2006 Aug 28;253(12):1572–80. doi:10.1007/s00415-006-0260-6
- ↑ Charcot-Marie-Tooth Disease Symptoms [Internet]. NHS; 2022 [cited 2023 May 8]. Available from: https://www.nhs.uk/conditions/charcot-marie-tooth-disease/symptoms/
- ↑ 12.0 12.1 12.2 12.3 Ivanovic V, Bjelica B, Palibrk A, Brankovic M, Bozovic I, Basta I, et al. Physical and Mental Aspects of Quality of Life in Patients With Charcot-Marie-Tooth Disease Type 1A. Front Neurol [Internet]. 2022 [cited 2023 May 11];13. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2022.852150
- ↑ Bellofatto M, Bertini A, Tramacere I, Manganelli F, Fabrizi GM, Schenone A, et al. Anxiety and depression in Charcot-Marie-Tooth disease: data from the Italian CMT national registry. J Neurol. 2023 Jan 1;270(1):394–401.
- ↑ 14.0 14.1 Carey IM, Nirmalananthan N, Harris T, DeWilde S, Chaudhry UAR, Limb E, et al. Prevalence of co-morbidity and history of recent infection in patients with neuromuscular disease: A cross-sectional analysis of United Kingdom primary care data. PLOS ONE. 2023 Mar 1;18(3):e0282513.
- ↑ 15.0 15.1 Kitani-Morii F, Noto Y ichi, Tsuji Y, Shiga K, Mizuta I, Nakagawa M, et al. Rate of Changes in CMT Neuropathy and Examination Scores in Japanese Adult CMT1A Patients. Front Neurol. 2020 Jul 16;11:626.
- ↑ 16.0 16.1 Mini Balance Evaluation Systems Test [Internet]. Shirley Ryan AbilityLab. 2013 [cited 2023 May 11]. Available from: https://www.sralab.org/rehabilitation-measures/mini-balance-evaluation-systems-test
- ↑ 17.0 17.1 Bellofatto M, Bertini A, Tramacere I, Manganelli F, Fabrizi GM, Schenone A, et al. Frequency, entity and determinants of fatigue in Charcot–Marie–Tooth disease. Eur J Neurol. 2023;30(3):710–8.
- ↑ Van Bloemendaal M, Bout W, Bus SA, Nollet F, Geurts AC, Beelen A. Validity and reproducibility of the Functional Gait Assessment in persons after stroke. Clin Rehabil. 2019 Jan;33(1):94–103.
- ↑ Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, Internal Consistency, and Validity of Data Obtained With the Functional Gait Assessment. Phys Ther. 2004 Oct 1;84(10):906–18.
- ↑ RxFunction. Healthcare Professionals – Mechanism of Action [Internet]. RxFunction; n.d. Available from: https://rxfunction.com/healthcare-professionals/
- ↑ RxFunction. Our Product – How walkasins works [Internet]. RxFunction; n.d. Available from: https://rxfunction.com/our-product/
- ↑ 22.0 22.1 22.2 22.3 McCorquodale D, Pucillo EM, Johnson NE. Management of Charcot-Marie-Tooth disease: improving long-term care with a multidisciplinary approach. Journal of multidisciplinary healthcare. 2016;9(1):7–19.
- ↑ 23.0 23.1 23.2 Oddsson LIE, Bisson T, Cohen HS, Iloputaife I, Jacobs L, Kung D, et al. Extended effects of a wearable sensory prosthesis on gait, balance function and falls after 26 weeks of use in persons with peripheral neuropathy and high fall risk—The walk2Wellness trial. Frontiers in aging neuroscience. 2022;14:931048–931048.
- ↑ Oddsson LIE, Bisson T, Cohen HS, Jacobs L, Khoshnoodi M, Kung D, et al. The Effects of a Wearable Sensory Prosthesis on Gait and Balance Function After 10 Weeks of Use in Persons With Peripheral Neuropathy and High Fall Risk - The walk2Wellness Trial. Frontiers in aging neuroscience. 2020;12:592751–592751.
- ↑ 25.0 25.1 25.2 25.3 Koehler-McNicholas SR, Danzl L, Cataldo AY, Oddsson LIE. Neuromodulation to improve gait and balance function using a sensory neuroprosthesis in people who report insensate feet - A randomized control cross-over study. PloS one. 2019;14(4):e0216212–e0216212.
- ↑ Hsu CL, Manor B, Iloputaife I, Oddsson LIE, Lipsitz L. Six month lower-leg mechanical tactile sensory stimulation alters functional network connectivity associated with improved gait in older adults with peripheral neuropathy – A pilot study. Frontiers in aging neuroscience. 2022;14:1027242–1027242.
- ↑ Joe Carlson. RxFunction aims to help patients with peripheral neuropathy get back on their feet [Internet]. Star Tribune; 2017. Available from: https://www.startribune.com/rxfunction-aims-to-help-patients-with-peripheral-neuropathy-get-back-on-their-feet/424465813/?refresh=true
- ↑ Piscosquito G, Reilly MM, Schenone A, Fabrizi GM, Cavallaro T, Santoro L, et al. Responsiveness of clinical outcome measures in Charcot−Marie−Tooth disease. Eur J Neurol. 2015;22(12):1556–63.
- ↑ A longitudinal study of CMT1A using Rasch analysis based CMT neuropathy and examination scores [Internet]. [cited 2023 May 11]. doi: 10.1212/WNL.0000000000009035.
- ↑ Modified Fatigue Impact Scale [Internet]. Shirley Ryan AbilityLab. 2012 [cited 2023 May 11]. Available from: https://www.sralab.org/rehabilitation-measures/modified-fatigue-impact-scale
- ↑ Functional Gait Assessment [Internet]. Shirley Ryan AbilityLab. 2016 [cited 2023 May 11]. Available from: https://www.sralab.org/rehabilitation-measures/functional-gait-assessment