Complications Post Amputation
Original Editor - Lauren Newcombe as part of the WCPT Network for Amputee Rehabilitation Project
Top Contributors - Sheik Abdul Khadir, Admin, Tarina van der Stockt, Tony Lowe, Kim Jackson, Aicha Benyaich, Lauren Lopez, Rachael Lowe, Naomi O'Reilly, Andeela Hafeez and Olajumoke Ogunleye
Introduction[edit | edit source]
As with any surgery, having an amputation carries a risk of complications. Surgeons will aim to reconstruct the limb to the best of their ability, taking into account soft tissue viability, bone length and other anatomical considerations. However, underlying disease state and post-operative management can result in complications, the most common of which are:
- Oedema
- Wounds and infection
- Pain
- Muscle weakness and contractures
- Joint instability
- Autonomic dysfunction
Learning outcomes
[edit | edit source]
At the end of this module, you should be able to:
- Understand the factors predisposing to succesfull and unsuccesful rehablitation.
- Have an wareness that pain (of the residuum, phantom pain) may affect the quality of life of the amputee.
- Understand the methods of pain relief for the post-operative treatment of phantom/senation pain.
Oedema[edit | edit source]
Stump oedema occurs as a result of trauma and the handling of tissues during surgery [1] . After amputation, there is an imbalance between fluid transfer across the capillary membranes and lymphatic reabsorption [2] . This, in combination with reduced muscle tone and inactivity, can lead to stump oedema.
The complications that can arise from stump oedema include wound breakdown, pain, reduced mobility and difficulties with prosthetic fitting .[3]
Numerous interventions are used across the country to manage and prevent stump oedema, including,compression socks, rigid removable dressings, exercise and PPAM aiding. The BACPAR post operative oedema guidance(2012) www.csp.org.uk/sites/files/csp/secure/guidance_v.8_0.pdf details the evidence behind these interventions and recommends the use of rigid removable dressings where expertise, time and resources allow.
The following video by Ossur shows an example of the application of a rigid removable dressing.
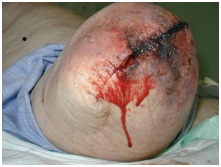
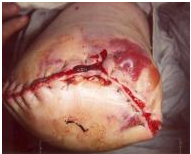
Wounds and Infection
[edit | edit source]
Surgical site infection after amputation is common and as well as increasing patient morbidity, can have negative effects on healing, phantom pain and time to prosthetic fitting [4] . Risk factors for a stump infection include diabetes mellitus, old age and smoking, which are all common denominators amongst the amputee population [5]. The decision to insert a drain and use clips instead of sutures is also associated with an increased infection risk.
Literature suggests a post-operative infection rate ranging from 12-70% in the UK [5] but this is widely due to the variation in the classification of stump wounds. The Centre for Disease Control (CDC) Surgical Site Infection (SSI) Criteria (2008) aims to make this classification more standardised:
| Superficial Incisional surgical site infection | Deep Incisional surgical site infection |
|---|---|
|
A Superficial Incisional surgical site infection must meet the following criterion :
|
A Deep Incisional surgical site infection meets the following criterion :
|
The potential consequences of infection include vac therapy, wound debridement and revision surgery. This can increase hospital length of stay and the risk of secondary morbidities such as pneumonia or reduced function. Wounds should be inspected regularly so that any signs of infection can be detected.
The following types of wounds may be encountered:
Tissue Necrosis[edit | edit source]
Poor tissue perfusion leads to ischaemia and necrosis. Dusky skin changes, mottled discolouration and slough can be observed. This can lead to subsequent wound breakdown and dehiscence [6]. Depending on the extent of non viable tissue, wound debridement or revision surgery is often necessary.
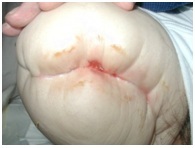
Skin Blisters[edit | edit source]
Wound oedema, reduced elasticity and tight stump dressings can all increase friction of the epidermis and cause blistering of the skin.
Sinus/Osteomyelitis[edit | edit source]
A deep, infected sinus can often mask osteomyelitis and delay healing. The sinus can extend from the skin to the subcutaneous tissues and management often includes aggressive antibiotic therapy. Sometimes, surgery is an option, however, this can impact on the shape of the stump and rehabilitation outcomes [6]
Wound management depends on the extent of non viable tissue. The following videos discuss wound classificationand common tissue viability techniques.
Wound Classification[edit | edit source]
Wound healing
www.oandp.org/olc/lessons/html/SSC_02/08wound.asp
Negative Pressure Therapy[edit | edit source]
Surgical Debridement
[edit | edit source]
Pain[edit | edit source]
Phantom limb pain is defined as "pain that is localised in the region of the removed body part" (Siddle, 2004) [7]. It is a poorly understood clinical phenomenon that remains the subject of intense research due to the acute and chronic nature of the condition. The incidence is reported to be as high as 60-80% in patients post amputation (D'arcy, 2005) [8] and risk factors include chronic pre-amputation pain, post-operative surgical painand psychological distress.
Phantom Limb Pain Video[edit | edit source]
There are numerous theories about the causes of phantom limb pain including peripheral, central and spinal theories.
Peripheral Theories[edit | edit source]
- Remaining nerves in the stump grow to form neuromas which generate impulses. These impulses are perceived as pain in the limb which has been removed.
- After changes in the severity of phantom limb pain were noted in different temperatures, another theory says that cooling of the nerve endings increases the rate of firing of the nerve impulses, which are perceived by the patient as phantom limb pain
Central Theories[edit | edit source]
- Melzack proposed that the body is represented in the brain by a matrix of neurons. Sensory experiences create a unique neuromatrix which is imprinted on the brain. When the limb is removed, the neuromatrix tries to reorganise but the neurosignature remains due to the chronic pain experienced prior to the amputation. This causes phantom limb pain after amputation.
Spinal Theories[edit | edit source]
- When peripheral nerves are cut during amputation, there is a loss of sensory input from the area below the level of amputation. This reduction in neurochemicals alters the pain pathway in the dorsal horn
Treatment
[edit | edit source]
Amitriptyline, gabapentin and other neuropathic painkillers are commonly used to treat phantom limb pain but as these don't work for everyone, a holistic approach to patient care is important and medication should be used in conjunction with other therapies [9].
Mirror box therapy, graded motor imagery, desensitisation exercises and acupuncture have all been used as effective treatments for phantom limb pain but there is little evidence to say whether one treatment is more beneficial than the other.
Mirror Box Therapy
[edit | edit source]
Graded Motor Imagery
[edit | edit source]
Muscle weakness, muscle contractures and joint instability[edit | edit source]
After amputation, it is not uncommon for patients to experience pain, muscle weakness or instability in structures not directly associated with the amputation. These compensatory structures are the muscles and joints that are required to perform additional functions post amputation, often resulting in stiffness, spasm or pain.
The effects of bed rest and reduced mobility are also well documented. Deconditioning results in diminished muscle mass, sarcomere shortening, reduced muscle strength and changes in cartilaginous structures [10] . It is therefore, crucial, that amputee patients undertake functional rehabilitation and personalised exercise programmes from as early as day 1 post surgery. Hip flexion contractures and knee flexion contractures are common complications post amputation and can impact significantly on prosthetic rehabilitation. Physiotherapy regimes should consist of the following elements:
- Range of movement exercises
- Strengthening exercises
- Stretches
- Core stability exercises
- Early mobility practice
- Transfer practice
- Balance exercises
- PPAM aiding and gait re-education
The following exercise regimes were developed by P.I.R.P.A.G for amputees and are used by many hospitals and limb centres across the United Kingdom.
www.irpag.org/PIRPAG
Autonomic Dysfunction[edit | edit source]
Complex regional pain syndromes (CRPS) are neuropathic pain disorders developing as a disproportionate consequence of trauma affecting the limbs [11]. Symptoms include distal pain, allodynia and autonomic and motor dysfunction. The residual limb can appear hot, swollen and trophic due to altered control of the sympathetic nervous system.
Due to the lack of understanding about the pathophysiological abnormalities underlying CRPS, treatment should be multi-disciplinary and comprise of neurologists, physiotherapists and psychologists to name but a few. Anti-depressants are proven to be beneficial in reducing neuropathic pain [12] alongside nerve blocks, TENS, graded exercise and mobilisation.
This lecture summarises our current understanding of CRPS:
References
[edit | edit source]
- ↑ BACPAR post operative oedema guidance 2012
- ↑ Airaksinen, O., Kolari, P.J., Herve, R. and Holopainen, R. (1988) Treatment of post- traumatic oedema in lower legs using intermittent pneumatic compression. Scandinavian Journal of Rehabilitation Medicine, 20(1), pp.25-28
- ↑ Engstrom, B and Van de Ven, C (1999). Therapy for Amputees. Churchill Livingstone.
- ↑ Coulston, J E, Tuff V, Twine C P, Chester J F, Eyers P S and Stewart A H R (2012) Surgical Factors in the Prevention of Infection Following Major Lower Limb Amputation. European Journal of Vascular and Endovascular Surgery, 43 (5), pp.556-560
- ↑ 5.0 5.1 Mcintosh J and Earnshaw J J (2009) Antibiotic Prophylaxis for the Prevention of Infection after Major Limb Amputation. European Journal of Vascular and Endovascular Surgery. 37 (6) pp.696-703
- ↑ 6.0 6.1 www.worldwidewounds.com
- ↑ Siddle L (2004) The challenge and management of phantom limb pain after amputation. British Journal of Nursing. Vol 13 (11) pp. 664-667
- ↑ Darcy Y (2005) Managing Phantom Limb Pain. www.nursing2005.com
- ↑ Fieldsen D (2011) Dealing with Phantom Limb Pain after Amputation. Nursing Times. Vol 107 (1) pp. 21-23
- ↑ Gillis A and Macdonald B (2005) Deconditioning in the hospitalised elderly. Canadian Nurse. Vol 101 (6)pp. 16-20
- ↑ Wasner G, Schattsneider J, Binder A and Baron R (2003) Complex regional pain syndrome-diagnostic mechanisms, CNS involvement and therapy. Spinal Cord. Vol 41. pp. 61-75
- ↑ Max MB et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992; 326: 1250