Parkinson's Pharmacotherapy: Difference between revisions
No edit summary |
No edit summary |
||
| Line 25: | Line 25: | ||
== Dopamine Agonist Medications == | == Dopamine Agonist Medications == | ||
Dopamine agonists | Dopamine receptor agonists came into the market for the treatment of PD in 1978. Dopamine agonists work by actively influencing dopamine receptors in the brain to produce more in-vivo dopamine, thus making it the preferential treatment early on in the disease process. <ref name=":8">Regina Katzenschlager MD, W. P. Apomorphine subcutaneous infusion in patients with Parkinson's with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet Neurology, 2018;(9):749-759. doi: 10.1016/S1474-4422(18)30239-</ref> Dopamine agonists are often prescribed as an initial therapy for PD, particularly in younger patients. This approach allows for a delay in the use of levodopa, which may reduce the impact of the problematic motor complications<ref name=":0" />. In general, dopamine agonists are not as potent as carbidopa/levodopa and may be less likely to cause dyskinesias. Examples include: Pramipexole (Mirapex®), Pramipexole Dihydrochloride Extended-Release (Mirapex ER®), Ropinirole (Requip®), Ropinirole Extended-Release Tablets (Requip® XL™). | ||
The main adverse effects seen after the intake of this medication include somnolence, withdrawal, and psychiatric disorders, such as confusion and hallucinations<ref name=":8" /><ref name=":9" />. It is vital that the physical therapist is aware of such side effects to dictate the treatment. | The main adverse effects seen after the intake of this medication include somnolence, withdrawal, and psychiatric disorders, such as confusion and hallucinations<ref name=":8" /><ref name=":9">Auffret M, D. S. (2018) Pharmacological Insights into the Use of Apomorphine in Parkinson's: Clinical Relevance. Clinical Drug Investigation, 2018; 38(4) 287-312. doi: 10.1007/s40261-018-0619-3.</ref>. It is vital that the physical therapist is aware of such side effects to dictate the treatment. | ||
It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist. | |||
== Anticholinergic Medications == | == Anticholinergic Medications == | ||
Revision as of 02:18, 15 April 2022
Introduction[edit | edit source]
Parkinson’s disease (PD) is a gradually progressive neurodegenerative condition. The etiology and pathogenesis remain incompletely understood. There are currently no disease-modifying treatments for PD, with no neuroprotective agents currently available, treatment should only be initiated when quality of life is affected, and the potential benefits and side effects of these drug classes should be discussed with the patient[1]. The long-term duration of disease means that patients may take sophisticated medication regimes aimed at controlling the motor symptoms, with a likelihood of problematic side effects.
Understanding the impact of medication on both the movement and thought quality of people with Parkinson’s will help set goals and plans for physiotherapy intervention.
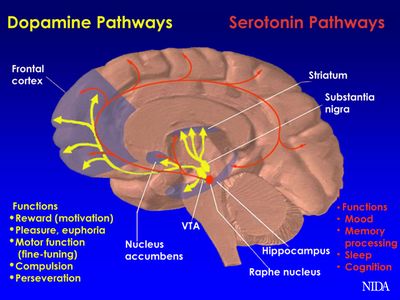
PD is caused by decreased dopamine production in the basal ganglia due to degeneration of dopamine-secreting neurons. Dopaminergic drugs designed to replace the action of dopamine in the deplete basal ganglia form the mainstay of PD treatment at present. This may be achieved through drugs that are metabolized to dopamine, that activate the dopamine receptor, or that prevent the breakdown of endogenous dopamine. Deep brain stimulation, stem cell therapy and gene therapy are alternative approaches that aim to lower the need to medications. Understanding the impact of medication on both the movement and thought quality of people with Parkinson’s will help set goals and plans for physiotherapy intervention.
The video below outlines briefly medication rational and major drug types
Main Medications[edit | edit source]
- Levodopa: The mainstay of current PD treatment are levodopa-based preparations, designed to replace the dopamine in the depleted striatum. See Levodopa in the Treatment of Parkinson's
- Dopamine agonists: Stimulate the activity of the dopamine system by binding to the dopaminergic receptors. Dopamine agonists are often prescribed as an initial therapy for PD, particularly in younger patients. This approach allows for a delay in the use of levodopa, which may reduce the impact of the problematic motor complications
- Drugs that prevent the breakdown of endogenous dopamine work by inhibiting the enzymes involved in dopamine metabolism, which preserves the levels of endogenous dopamine eg Monoamine Oxidase B (MAO-B) inhibitors, Catechol-O-methyl transferase inhibitors.
- Anticholinergics: reduce the activity of the neurotransmitter acetylcholine, by acting as antagonists at cholinergic receptors. While their role is limited and they are now prescribed infrequently, they may offer some benefit in improving rigidity and tremor in PD
Levodopa[edit | edit source]
The mainstay of current PD treatment are levodopa-based preparations, designed to replace the dopamine in the depleted striatum. Dopamine itself is unable to cross the blood brain barrier (BBB) and cannot be used to treat PD. In contrast, the dopamine precursor levodopa is able to cross the BBB and can be administered as a therapy. After absorption and transit across the BBB, it is converted into the neurotransmitter dopamine by DOPA decarboxylase
Prevention of the Breakdown of Endogenous Dopamine[edit | edit source]
MAO-B Inhibitors work by inhibiting the enzymes involved in dopamine metabolism, which preserves the levels of endogenous dopamine eg Monoamine Oxidase B (MAO-B) inhibitors, Catechol-O-methyl transferase inhibitors. While they are sometimes sufficient for control of symptoms in early disease, most patients ultimately require levodopa-based treatment. MAO-B inhibitors may also be used in combination with levodopa-based preparations, to allow for a reduction in the levodopa dose. Commonly used MAO-B inhibitors include selegiline (Deprenyl, Eldepryl, Zelapar) and rasagiline (Azilect). More recently, the drug safinamide (Xadago) was also approved for use in PD, which appears to have multiple modes of action, one of which is thought to be inhibition of MAO-B [3][4]. MAO-B inhibitors are generally well tolerated, with gastrointestinal side effects being the most common problem. Other adverse effects include aching joints, depression, fatigue, dry mouth, insomnia, dizziness, confusion, nightmares, hallucinations, flu-like symptoms, indigestion, and headache.[3]
Catechol-O-methyl transferase inhibitors: another enzyme that is involved in dopamine degradation is COMT. These drugs are predominantly used as adjunctive therapy to levodopa, prolonging its duration of action by increasing its half-life and its delivery to the brain. COMT inhibitors come in the form of tablets and are not generally prescribed as monotherapy, as on their own they offer only limited effect on PD symptoms. Examples of COMT inhibitors include entacapone (Comtan), tolcapone (Tasmar), and opicapone (Ongentys). [3]
Dopamine Agonist Medications[edit | edit source]
Dopamine receptor agonists came into the market for the treatment of PD in 1978. Dopamine agonists work by actively influencing dopamine receptors in the brain to produce more in-vivo dopamine, thus making it the preferential treatment early on in the disease process. [5] Dopamine agonists are often prescribed as an initial therapy for PD, particularly in younger patients. This approach allows for a delay in the use of levodopa, which may reduce the impact of the problematic motor complications[3]. In general, dopamine agonists are not as potent as carbidopa/levodopa and may be less likely to cause dyskinesias. Examples include: Pramipexole (Mirapex®), Pramipexole Dihydrochloride Extended-Release (Mirapex ER®), Ropinirole (Requip®), Ropinirole Extended-Release Tablets (Requip® XL™).
The main adverse effects seen after the intake of this medication include somnolence, withdrawal, and psychiatric disorders, such as confusion and hallucinations[5][6]. It is vital that the physical therapist is aware of such side effects to dictate the treatment.
It acts as a nicotinic antagonist, dopamine agonist, and noncompetitive NMDA antagonist.
Anticholinergic Medications[edit | edit source]
The medications that have so far been discussed are all aim to increase dopaminergic activity in the striatum. These reduce the activity of the neurotransmitter acetylcholine, by acting as antagonists at cholinergic receptors. While their role is limited and they are now prescribed infrequently, they may offer some benefit in improving rigidity and tremor in PD. Loss of dopaminergic neurons results in disturbance of the normal balance between dopamine and acetylcholine in the brain, and anticholinergic drugs may lead to restoration and maintenance of the normal balance between these two neurotransmitters.[3]
The main role of these drugs is in young patients at early stages of the disease for the relief of mild movement symptoms, particularly tremors and muscle stiffness. They are generally avoided in elderly patients or those with cognitive problems, due to an increased risk of confusion with this class of drugs[3].
Examples of anticholinergics include benztropine, orphenadrine, procyclidine, and trihexyphenidyl (Benzhexol). Common adverse effects of anticholinergic drugs include memory problems, drowsiness, constipation, sedation, urinary retention, blurred vision, tachycardia, and delirium. Increased side effects are typically seen in the elderly, when compared with younger adults. As a physical therapist, these are all things to consider, especially when treating your elderly patients[7].
Conclusion[edit | edit source]
Levodopa, MAO-B inhibitors, Dopamine agonist, and Anticholinergic drugs are the main medications used in the treatment of the neurodegenerative condition, Parkinson’s Disease. Understanding the importance and impact of antiparkinsonian medications on our patients living with this disease is imperative. Being able to recognize early warning signs of adverse symptoms of the medications such as: weakness, dizziness, confusion, and dyskinesias could greatly alter our plan of care and the patient’s safety. If left unaddressed, these adverse effects could substantially decrease our overall quality of care that we could administer. Furthermore, if we had a better grasp on how the drug worked within the body from the time it was given, to the point of excretion, a physical therapist may be able to plan their time of care accordingly to avoid these adverse issues.
References[edit | edit source]
- ↑ Pharmaceutical Journal Parkinson’s disease: management and guidance Available: https://pharmaceutical-journal.com/article/ld/parkinsons-disease-management-and-guidance(accessed 14.4.20220
- ↑ PD care New York Taking Control: Medications for Parkinson's Available from: https://www.youtube.com/watch?v=T8VojsSvv4E (last accessed 8.11.2019)
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Zahoor I, Shafi A, Haq E. Pharmacological treatment of Parkinson’s disease. Exon Publications. 2018 Dec 21:129-44. Available:https://www.ncbi.nlm.nih.gov/books/NBK536726/(accessed 14.4.2022)
- ↑ Teo KC, Ho SL. Monoamine oxidase-B (MAO-B) inhibitors: implications for disease-modification in Parkinson’s disease. Translational neurodegeneration 2013 Dec;2(1):19.
- ↑ 5.0 5.1 Regina Katzenschlager MD, W. P. Apomorphine subcutaneous infusion in patients with Parkinson's with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet Neurology, 2018;(9):749-759. doi: 10.1016/S1474-4422(18)30239-
- ↑ Auffret M, D. S. (2018) Pharmacological Insights into the Use of Apomorphine in Parkinson's: Clinical Relevance. Clinical Drug Investigation, 2018; 38(4) 287-312. doi: 10.1007/s40261-018-0619-3.
- ↑ Brocks, D. R. Anticholinergic drugs used in Parkinson's: An overlooked class of drugs from a pharmacokinetic perspective. J Pharmaceut Sci.https://sites.ualberta.ca/~csps/JPPS2(2)/D.Brocks2/anticholinergic.htm. August 22, 1999. Accessed November 5, 2018