Psychological Basis of Pain: Difference between revisions
Evan Thomas (talk | contribs) mNo edit summary |
Kim Jackson (talk | contribs) m (Text replacement - "[[Fear‐Avoidance Belief Questionnaire" to "[[Fear Avoidance Belief Questionnaire") |
||
| (47 intermediate revisions by 12 users not shown) | |||
| Line 6: | Line 6: | ||
== Introduction == | == Introduction == | ||
[[File:Pain stress brain.png|thumb]] | |||
The International Association for the Study of Pain '''(IASP)''' definition of pain highlights the multidimensional components of pain. The interaction of these dimensions ('''sensory-discriminative:''' intensity, location, quality and behaviour of pain; '''cognitive-evaluative:''' thoughts of the pain as influenced by previous experiences and knowledge; and '''motivational-affective''': emotional responses like anger, anxiety and fear that motivate the response to pain)<ref name="Gifford and Butler">Gifford, LS.Butler, D.S. The Integration of Pain Sciences into Clinical Practice. Journal of Hand Therapy 1997; 10: 86-95</ref> all contribute to the complexity of the painful experience. An individual’s belief systems, pain understanding, thoughts and emotions: anxiety; depression; catastrophizing (or their psychology) influence how the brain interprets a noxious stimulus in relation to the meaning and level of threat the stimulus pose to one's well-being, and thereby influence the resulting output from the brain<ref>Miller RM, Kaiser RS. Psychological characteristics of chronic pain: a review of current evidence and assessment tools to enhance treatment. Current pain and headache reports. 2018 Mar 1;22(3):22.</ref>. Such resulting changes in behaviour (i.e. avoidance)<ref name="Moseley">Moseley GL. A pain neuromatrix approach to patients with chronic pain. Manual Therapy 2003 doi:10.1016/S1356-689X(03)00051-1</ref><ref name="Turk and Okifugi">Turk D, Okifugi A. Psychological Factors in chronic pain: evolution and revolution. J of Consulting and Clinical Psychology 2002; 70: 678-690</ref> such as fear avoidance, catastrophizing, pain-related anxiety, stress and learned helplessness can impact upon a pain experience and a patient's coping strategies. While this is unlikely to be an exclusive list of influential psychosocial factors, the following components are well researched and evidenced. This page will review each of these in turn. | |||
== Fear Avoidance == | |||
== Fear avoidance | [[Psychological approaches to pain management|'''Pain and Fear''']]: Fear is a typical emotional response to an experience that is perceived to be threatening, such as one that leads to a pain sensation<ref name="Rainville et al">Rainville J, Smeets RJEM, Bendix T, Tveito TH, Poiraudeau S, Indahl AJ. Fear-avoidance beliefs and pain avoidance in low back pain- translating research into clinical practice. The Spine Journal 2011; 11: 895-903</ref><ref name="Leeuw et al">Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The Fear-Avoidance Model of Musculoskeletal Pain: Current State of Scientific Evidence. Journal of Behavioural Medicine 2007; 30: 77-94</ref>. Future avoidance of the painful activity is often the resulting behaviour adopted to protect oneself from a repeat of the fearful emotion and painful experience. This pattern can lead to fear and avoidance of work-related activities, movement, and re-injury<ref name="Vlaeyen and Linton">Vlaeyen JWS and Linton SJ. Fear –avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000; 85: 317-332</ref>. '''''Kinesophobia''''' is a term used interchangeably with fear avoidance in health and pain literature. It is defined as “an excessive, irrational, and debilitating fear of physical movement and activity resulting from a feeling of vulnerability to painful injury or reinjury<ref name="Kori et al">Kori, S.H., Miller, R.P., Todd, D.D. Kinesophobia: A new view of chronic pain. Pain Management 1990; 3:35-43</ref>.” | ||
=== '''Impact of Fear Avoidance on the Pain Experience''' === | |||
[[File:Fear-avoidance-picture.jpg|thumb]] | |||
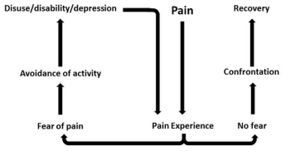
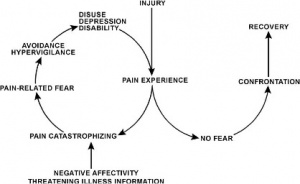
In response to acute tissue damage/injury, feelings of fear are heightened as part of a normal consequence of pain<ref name="Turk and Okifugi" />. The individual will rest and protect the painful area as an adaptive behaviour to allow tissue healing to occur<ref name="Turk and Okifugi" />. As the acute phase and initial tissue healing resolves, it has been suggested that some individuals can confront their fearful emotions and are able to resume normal activities, which ultimately helps extinguish their fears as they experience positive increase in mobility/movement unassociated with further increases in pain<ref name="Rainville et al" />. For others, they may be unable to '''overcome the fearful emotion''' and the resulting '''avoidance behaviour persists'''<ref name="Vlaeyen and Linton" />. A cycle of continued activity avoidance and fearful emotions may ensue, resulting in chronic pain that may have an adverse effect on the musculoskeletal and cardiovascular system (disuse syndrome)<ref name="Bortz">Bortz, WM. The disuse syndrome: West J Med 1984; 41: 691-694</ref>, which fundamentally increases disability<ref name="Turk and Okifugi" /><ref name="Vlaeyen and Linton" /><ref>Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000 Apr 1;85(3):317-32.</ref>. | |||
'''Several models have been proposed to explain the persistence of 'fear-avoidance'''' '''and its impact on pain and behaviour'''. | |||
In | In the '''[[Fear Avoidance Model|fear-avoidance model of pain]]''', individuals who are likely to demonstrate ongoing fear-avoidance behaviours will be inclined to '''catastrophize'''<ref name="Leeuw et al" /><ref name="Vlaeyen and Linton" />: | ||
[[File:Pain fear.jpg|center|thumb]] | |||
<br> | |||
Vlaeyen and Linton<ref name="Vlaeyen and Linton" /> have described the interconnections contributing to fear avoidance as follows: with negative appraisals of a painful experience, fear avoidance behaviours are elicited. Due to hypervigilance, the avoidance behaviour begins to occur in anticipation rather than in response to pain. As there are fewer and fewer opportunities to correct the wrongful association of pain with the avoided activity, the behaviour perpetuates and fear avoidance persists.<br> | |||
While research has consistently shown an association between fear avoidance behaviour and pain-related fears<ref>Linton SJ. A Review of Psychological Risk Factors in Back and Neck pain. Spine 2000; 25: 1148-1156</ref><ref name="Boersma and Linton">Boersma K, Linton SJ. How does persistent pain develop? An analysis of the relationship between psychological variables, pain and function across stages of chronicity. Behav Res Ther 2005; 43: 1495-1507</ref><ref>Boersma K, Linton SJ. Expectancy, fear and pain in the prediction of chronic pain and disability: A prospective analysis. Europ J of Pain 2006; 10: 551-557</ref><ref>Grotle M, Vollestad NK, Veierod MB, Brox JI. Fear-avoidance beliefs and distress in relation to disability in acute and chronic back pain. Pain 2004; 112: 343-352.</ref><ref>Picavet HS, Vlaeyen JWS, Schouten JS. Pain catastrophizing and kinesophobia: predictors of chronic back pain. Am J Epidemiol 2002; 156: 1028-1034</ref> even when pain intensity and other variables were controlled in patients with chronic pain, there are conflicting studies about this association in patients with acute pain. '''Studies have shown that high levels of fear avoidance''' can be '''a predictor for future episodes of pain'''<ref>Linton SJ, Buer N, Vlaeyen JWS, Hellsing, AL. Are fear –avoidance beliefs related to the inception of an episode of back pain? A prospective study. Psychol Helath 1999; 14: 1051-1059</ref><ref>Klenerman ChM, Slade PD, Stanley IM, Pennie B, Reilly JP, Atchinsn LE, Troup JDG, Rose MJ. The Prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine 20; 4: 478-484</ref><ref>Fritz JM, George SZ, Delitto A. The role of fear-avoidance beliefs in acute low back pain: relationships with current and future disability work status. Pain 2001; 94: 7-15</ref><ref>Swinkels-Meewise IE, Roelofs J, Verbeek AL, Oostendorp RA, Vlaeyen JWS. Fear of movement/(re)injury, disability and participation in acute low back pain. Pain 2003; 105: 371-379</ref><ref>Swinkels-Meewise IE, Roelofs J, Oostendorp RA, Verbeek AL, Vlaeyen JWS. Acute low back pain: pain-related fear and catastrophizing influence physical performance and perceived disability. Pain 2006; 120: 36-43</ref>. But on the contrary, studies<ref>Sieben JM, Portegijs PJM, Vlaeyen JWS, Knottnerus JA. Pain related fear at the start of a new low back pain episode. European J Pain 2005; 9: 635-641</ref> showed more support for the link between [[Depression|depression]] with poor function and disability at this early stage<ref name="Pincus et al">Pincus T, Vogel S, Burton AK, Santos R, Field A. Fear avoidance and prognosis in back pain. A systematic review and synthesis of current evidence. Arthritis and Rheumatism 2006; 54: 3999-4010</ref><ref>Boersma K, Linton SJ. How does persistent pain develop? An analysis of the relationship between psychological variables, pain and function across stages of chronicity. Behav Res Ther 2005; 43: 1495-1507</ref> In the '''learning pathway model,''' fear avoidance becomes a conditioned response through association of pain with movement<ref name="Vlaeyen and Linton" /><ref name="Rainville et al" /><ref name="Pincus et al" />. The behaviour of avoiding expected pain-provoking movement or activities to prevent new episodes of pain is learned through experience<ref>Fordyce WE, Shelton JL, Dundore DE. The modification of avoidance learning in pain behaviors. J Behav Med 1982; 5: 405-414</ref>. The individual may anticipate similar experiences and associate non-painful or non-harmful experiences with pain, resulting in persistent fear-avoidance behaviour<ref name="Vlaeyen and Linton" /><ref name="Pincus et al" />. <br> | |||
The following '''factors''' can affect '''the strength of association of pain with movement and the fear-avoidance behaviour''' that patients may exhibit:<ref>Turk DC, Monarch ES. Biopsychosocial perspective on chronic pain. Psychological approaches to pain management: A practitioner's handbook. 1996:3-2.</ref> | |||
*existing beliefs about pain and the meaning of the symptoms | *existing beliefs about pain and the meaning of the symptoms | ||
*beliefs about their own ability to control pain (self-efficacy) | *beliefs about their own ability to control pain '''(self-efficacy)''' | ||
*prior learning experiences (either direct experience or observational learning) | *prior learning experiences (either direct experience or observational learning) | ||
*expectations of recovery | *expectations of recovery | ||
*current emotional states (which include catastrophizing, anxiety, depression) | *current emotional states ('''which include catastrophizing, anxiety, depression''') | ||
While the above models | While the above models explain how fear avoidance becomes a '''maladaptive coping strategy''' and barrier to recovery in patients with chronic pain, this information may also be relevant to clinicians dealing with patients in the acute stages of injury. Any early fear-avoidance behaviours, especially for those who have strong negative emotions about their pain like anxiety or depression<ref name="Boersma and Linton" /><ref name="Pincus et al" />, could be identified. These patients could be monitored so they do not continue this coping strategy once the acute stage has passed. Knowing what factors affect fear-avoidance would help clinicians elicit these behaviours in their patients, expose any wrongful beliefs, attitudes or coping strategies (active vs passive) patient may have about their pain and recovery, and determine the best way to challenge them. | ||
Physiotherapists managing these patients may find it useful to categorise | Physiotherapists managing these patients may find it useful to categorise the type of fear-avoidance patients exhibit. '''Rainville et a'''l<ref name="Rainville et al" /> proposed the following categories: | ||
* | |||
* | *'''Misinformed avoiders:''' have misinformed beliefs about movement and risk of damage or re-injury; may benefit from accurate information about pain and movement (pain physiology) | ||
* | *'''Learned avoiders:''' learned the behaviour without being aware nor overly distressed about it; may benefit from [[Exercise and Activity in Pain Management|gradual exposure to activity depending]] on pain tolerance | ||
*'''Affective avoiders:''' are distressed and have strong negative cognitions about the pain; may need more [[Psychological basis of pain|cognition-based management]] and challenge beliefs | |||
While not every patient with chronic pain will fall into any specific model or category, presenting an appropriate model may help the patient to realise the cycle of pain and fear-avoidance behaviour that individual has adopted. Furthermore, explaining how fear avoidance affects the physiological feelings they may be experiencing will help them to understand how their perception of pain may be altered<ref name="Turk and Okifugi" /><ref name="Vlaeyen and Linton" /><ref name="Leeuw et al" />. For example: | |||
*Increased physiological arousal/heightened muscle reactivity | *Increased physiological arousal/heightened muscle reactivity | ||
* | *Associated anxiety and hyper-vigilance leads to increased attention to expected painful stimulus which in itself can intensify the pain | ||
*Reduced participation in valued activities like work, leisure and social contacts can lead to mood changes like irritability, frustration and depression | *Reduced participation in valued activities like work, leisure and social contacts can lead to mood changes like irritability, frustration and depression; these mood changes could further affect perception of pain | ||
*Disuse or disability from persistent avoidance behavior will | *Disuse or disability from persistent avoidance behavior will lead to pain with much less provocation than before (lower the threshold for experiencing future pain). | ||
Several self-reported measures of fear avoidance could be used in the clinic to help assess fear avoidance. These include the following: | Several '''self-reported measures''' of fear avoidance could be used in the clinic to help assess fear avoidance. These include the following: | ||
#[[Fear Avoidance Belief Questionnaire|Fear-Avoidance Beliefs Questionnaire (FABQ]]) identifies how beliefs about work and physical activity affect low back pain<ref name="Waddell et al">Waddell G, Newton M, Henderson I, Somerville D, Main C. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance in chronic low back pain and disability. Pain 1993; 52: 157-168</ref> | |||
#Survey of Pain Attitudes (SOPA) assesses patients attitudes towards pain control, pain-related disability, medical cures of pain, solicitude of others, and medication for pain<ref name="Jensen et al">Jensen MP, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients' attitudes toward pain. J Psychosom Res 1987; 31: 393-400</ref> | |||
#Tampa Scale for Kinesiophobia (TSK) assesses fear of re-injury due to movement<ref name="Kori et al" /> | |||
== Anxiety and Depression == | == Anxiety and Depression == | ||
'''Anxiety''' | === '''Anxiety''' === | ||
*Anxiety is a general term for several disorders that include uneasiness, apprehension, nervousness, fear and worrying. | |||
*Anxiety is | *There is high prevalence of anxiety reported throughout the literature in patients with chronic pain syndromes of varying nature including the following: non-specified/widespread chronic pain<ref>Valente MA, Ribeiro José, Jensen MP. Coping, Depression, Anxiety, Self-Efficacy and Social Support: Impact on Adjustment to Chronic Pain. Escritos de Psycologia; Vol 2 No.3 (2009): 8-17</ref><ref>Tekur P, Chatametcha S, Hankey A, Negandra HR and Nagaranthna R. A comprehensive yoga programs improves pain, anxiety and depression in chronic low back pain patients more than exercise: An RCT. Complementary Medicines Therapy; Vol 20 Issue 3 (2012): 107-118</ref><ref>Castro MMC and Daltro C. Sleep patterns and symptoms of anxiety and depression in patients with chronic pain. Arquivos de Neuropsiquiatria 67, 1 (2009): 25-28</ref><ref>Castro MMC, Quarantini LC, Daltro C, Pires-Caldas M, Koenan KC, Kraychete DC and de Oliveira I. Comorbid depression and anxiety symptoms in chronic pain patients and their impact on health-related quality of life. Revista de Psyquiatria Clinica; 38, 4 (2011): 126-129</ref><ref name=":1" /><ref name=":2" />, low back pain<ref>Bener A, Verjee M, Defeeah EE, Falah O, Al-Jushaishi T, Schlogl J, Sedeeq A and Khan S. Psychological factors: anxiety, depression, and somatization symptoms in low back pain patients. Journal of Pain Research; 6 (2013): 95-101</ref>, traumatic musculoskeletal injury (TMsI)<ref>Rosenbloom BN, Khan S, McCartney C and Katz J. Systematic review of persistent pain and psychological outcomes following traumatic musculoskeletal injury. Journal of Pain Research; 6 (2013): 39-51</ref><ref>Castillo RC, Wegener ST, Heins SE, Haythornthwaite JA, MacKenzie EJ and Bosse MJ. Longitudinal relationships between anxiety, depression, and pain: Results from a two-year cohort study of lower extremity trauma patients. Pain; 154, 12 (2013): 2860-2866</ref>, rotator cuff tear <ref>Potter MQ, Wylie JD, Greis PE, Burks RT and Tashjian RZ. Psychological Distress Negatively Affects Self-assessment of Shoulder Function in Patients With Rotator Cuff Tears. Clinical Orthopaedics and Related Research; 472, 12 (2014): 3926-3932</ref>; and also in non-musculoskeletal (MSK) conditions such as phantom limb pain <ref>Kazemi H, Ghassemi S, Fereshtehnejad SM, Amini A, Kolivand PH and Doroudi T. Anxiety and Depression in Patients with Amputated Limbs Suffering from Phantom Pain: A Comparative Study with Non-Phantom Chronic Pain. International Journal of Preventative Medicine; 4, 2 (2013): 218-225</ref>, prostatitis/chronic pelvic pain syndrome<ref>Ahn SG, Kim SH, Chung KI, Park KS, Cho SY and Kim HW. Depression, Anxiety, Stress Perception, and Coping Strategies in Korean Military Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Korean Journal of Urology; 53, 9 (2012): 643-648</ref> and diabetic neuropathy<ref>Jain R, Jain S, Raison CL and Maletic V, “Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators”, Current Diabetes Reports; 11, 4 (2011): 275-284</ref>. | ||
*There is high prevalence of anxiety reported throughout the literature in patients with chronic pain syndromes of varying nature including | |||
*The prevalence of patients in each study purely with anxiety varied throughout, from 18 to 72% of those recruited. Unfortunately much of this literature does not solely focus on anxiety alone. | *The prevalence of patients in each study purely with anxiety varied throughout, from 18 to 72% of those recruited. Unfortunately much of this literature does not solely focus on anxiety alone. | ||
*The following have been commonly reported anxiety symptoms reported in patients with chronic pain | *The following have been commonly reported anxiety symptoms reported in patients with chronic pain: “not being able to stop worrying,” “worrying about too many different things” and “feeling as though something awful might happen.” | ||
*A study conducted by Asmundson and Taylor in 1996 suggested the relation between anxiety sensitivity and developing pain-related fear and escape/avoidance in people with chronic pain<ref>Asmundson GJ, Taylor S. Role of anxiety sensitivity in pain-related fear and avoidance. Journal of behavioral medicine. 1996 Dec 1;19(6):577-86.</ref>. | |||
A study which aimed to evaluate the prediction of chronic pain looked at anxiety, depression and social stressors as risk factors and the severity of pain at 3, 6 and 12 months in 250 patients. They found a strong correlation between baseline anxiety which predicted pain severity at 12 months. Interestingly no correlation was found for social stressors or depression. In a TMsI study, anxiety was found to predict pain intensity at different time-frames post injury whereas depression, social support, length of hospital stay and self-efficacy had no substantial effect. They summarised that pain predicted anxiety and depression in the first year, but anxiety only predicted pain intensity from 12-24 months. Anxiety symptoms were therefore hypothesised as the primary causative factor of persistent pain in this cohort study. It is therefore important to address patients anxiety early to prevent it persisting and causing a negative barrier to their recovery.<br> | A study<ref name=":1">Bair MJ, Poleshuck EL, Wu J, Krebs EK, Damush TM, Tu W, Kroenke K. Anxiety but not social stressors predict 12-month depression and pain severity. ''Clin J Pain''. 2013 Feb;29(2):95-101. doi: 10.1097/AJP.0b013e3182652ee9.</ref> which aimed to evaluate the prediction of chronic pain looked at anxiety, depression and social stressors as risk factors and the severity of pain at 3, 6 and 12 months in 250 patients. They found a strong correlation between baseline anxiety which predicted pain severity at 12 months. Interestingly no correlation was found for social stressors or depression. In a TMsI study, anxiety was found to predict pain intensity at different time-frames post injury whereas depression, social support, length of hospital stay and self-efficacy had no substantial effect. They summarised that pain predicted anxiety and depression in the first year, but anxiety only predicted pain intensity from 12-24 months. Anxiety symptoms were therefore hypothesised as the primary causative factor of persistent pain in this cohort study. It is therefore important to address patients anxiety early to prevent it persisting and causing a negative barrier to their recovery.<br> | ||
One study examined the validity of a single question to screen for depression and anxiety<ref>Reme SE, Lie SA and Eriksen HR. Are 2 Questions Enough to Screen for Depression and Anxiety in Patients With Chronic Low Back Pain? Spine journal 39; 7 (2014) 455-462, doi:10.1097/BRS.0000000000000214, Accessed 06/11/14.</ref>. | One study examined the validity of a single question to screen for depression and anxiety<ref>Reme SE, Lie SA and Eriksen HR. Are 2 Questions Enough to Screen for Depression and Anxiety in Patients With Chronic Low Back Pain? Spine journal 39; 7 (2014) 455-462, doi:10.1097/BRS.0000000000000214, Accessed 06/11/14.</ref>. | ||
* | *Single question screening tools were compared with validated questionnaires such as mini-international neuropsychiatric interview, Hospital Anxiety and Depression Scale (HADS) and Hopkins symptom checklist. | ||
* | *It was found that the single questions demonstrated fair ability to detecting anxiety with a sensitivity of 68% and a good ability to exclude anxiety with a specificity of 85%. | ||
*Therefore single question screening tools are fairly effective in identifying anxiety and could be utilised into early assessments with chronic pain patients. <br> | *Therefore, single question screening tools are fairly effective in identifying anxiety and could be utilised into early assessments with chronic pain patients. <br> | ||
=== '''Depression''' === | |||
Depression is described as a general term for mental disorders which include: sadness, loss of interest or pleasure, feelings of low self-worth and low mood. | |||
A | *Some frequently reported depressive symptoms among chronic pain patients are: “thinking of suicide/self harm” and “feeling down, depressed or hopeless”. | ||
*Depression alone is associated with: chronic MSK<ref>Akkaya N, Atalay NŞ, Konukcu S and Şahin F. Effect of Number of Physical Therapy Sessions on Level of Pain, Depression, Anxiety, and Quality of Life. Journal Physical Medicine & Rehabilitation; 16 (2013): 8-13</ref> and widespread pain, knee pain<ref>Phyomaung PP, Dubowitz J, Cicuttini FM, Fernando S, Wluka AE, Raaijmaakers P, Wang Y and Urquhart D. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Musculoskeletal Disorders; 15, 10 (2014), doi:10.1186/1471-2474-15-10, Accessed 01/11/14.</ref>, low back pain and chronic pelvic pain/prostatitis<ref>Tripp DA, Nickel JC and Katz L. A feasibility trial of a cognitive-behavioural symptom management program for chronic pelvic pain for men with refractory chronic prostatitis/chronic pelvic pain syndrome. Canadian Urological Association Journal; 5, 5 (2011), doi:10.5489/cuaj.10201, Accessed 05/11/14</ref>. | |||
*In a study examining the impact of anxiety and depression in phantom limb pain (PLP) patients, mean depression scores were higher, although non-significant, in patients with non-PLP chronic pain syndromes<ref>Elbinoune I, Amine B, Shyen S, Gueddari S, Abouqal R, Hajjaj-Hassouni N. Chronic neck pain and anxiety-depression: prevalence and associated risk factors. Pan Afr Med J. 2016 May 27;24:89. doi: 10.11604/pamj.2016.24.89.8831.</ref>. | |||
*Similarly, a cohort of 400 patients with chronic myofascial or neuropathic pain demonstrated higher prevalence of depression (93%) than anxiety (72%), | A study of low back pain observed higher prevalence of depression symptoms (13.7%) compared to anxiety symptoms (9.5%)<ref>Bener A, Verjee M, Dafeeah EE, Falah O, Al-Juhaishi T, Schlogl J, Sedeeq A, Khan S. Psychological factors: anxiety, depression, and somatization symptoms in low back pain patients. ''J Pain Res''. 2013;6:95-101. doi: 10.2147/JPR.S40740.</ref>. | ||
*Similarly, a cohort of 400 patients with chronic myofascial or neuropathic pain demonstrated higher prevalence of depression (93%) than anxiety (72%)<ref>Castro Martha MC, Daltro C. Sleep patterns and symptoms of anxiety and depression in patients with chronic pain. Arq. Neuro-Psiquiatr. 2009 Mar;67(1): 25-28. Available from:<nowiki>https://doi.org/10.1590/S0004-282X2009000100007</nowiki>.</ref>. | |||
'''Pain, Anxiety and Depression''' Anxiety and depression appear to be overlapping conditions. Much of the literature has acknowledged their joint effect in chronic pain patients<ref>McBeth J, McFarlane GJ, Hunt IM and Silman AJ. Risk factors for persistent chronic widespread pain: a community‐based study. Rheumatology; 40 (2001), doi: 10.1093/rheumatology/40.1.95, Accessed 10/11/14. | '''Pain, Anxiety and Depression''' Anxiety and depression appear to be overlapping conditions. Much of the literature has acknowledged their joint effect in chronic pain patients<ref>McBeth J, McFarlane GJ, Hunt IM and Silman AJ. Risk factors for persistent chronic widespread pain: a community‐based study. Rheumatology; 40 (2001), doi: 10.1093/rheumatology/40.1.95, Accessed 10/11/14. </ref>.<br> | ||
A | A study in Brazil, which included 400 chronic pain patients, observed 54% with both of the psychiatric disorders compared to anxiety (18%) or depression (7%) alone<ref>Castro Martha MC, Quarantini Lucas C, Daltro C, Pires-Caldas M, Koenen KC, Kraychete DC, de Oliveira IR. Comorbid depression and anxiety symptoms in chronic pain patients and their impact on health-related quality of life. Rev. psiquiatr. clín. 2011;38(4):126-129. Available from:<nowiki>https://doi.org/10.1590/S0101-60832011000400002</nowiki>.</ref>. In patients with chronic musculoskeletal pain, those with both anxiety and depression experienced the greatest pain severity, a highly significant finding. Furthermore combined psychiatric co-morbidity was strongly associated with disability days with the following: 18.1 days in pain-only patients, 32.2 in those with pain and anxiety, 38.0 days in those with pain and depression and 42.6 days in those with all three conditions. This study also found a significant correlation with reduced QoL when all three conditions presented together<ref name=":2">Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008 Oct;70(8):890-7. doi: 10.1097/PSY.0b013e318185c510..</ref>. | ||
<br>Thus it comes into question as to why these psychiatric disorders: <br>a) present together,<br>b) develop with the onset of traumatic pain,<br>c) appear to cause acute pain to persist; and<br>d) cause more severe functional deficit together than when presenting independently. | |||
Many studies have looked into the pathophysiology of pain, anxiety and depression to find answers and to facilitate more successful treatment of these complex conditions<ref>Bair MJ, Robinson RL, Katon W and Kroenke K. Depression and Pain Comorbidity: A Literature Review. ''Arch Intern Med.'' 2003;163(20):2433–2445. doi:10.1001/archinte.163.20.2433</ref><ref>Urits I, Burshtein A, Sharma M, Testa L, Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B and Kaye AD''.'' Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. ''Curr Pain Headache Rep.'' 2019;23(23). Available from: <nowiki>https://doi.org/10.1007/s11916-019-0757-1</nowiki></ref><ref>de Heer E, Gerrits M, Beekman A, Dekker J, van Marwijk H, de Waal M et al. The Association of Depression and Anxiety with Pain: A Study from NESDA. PLoS ONE. 2014;9(10):e106907. </ref>. | |||
== Impact of Depression and Anxiety on the Pain Experience == | |||
''' | |||
=== Anxiety === | |||
A physiological model of anxiety may explain its role in pain perception; | A physiological model of anxiety may explain its role in pain perception; | ||
*It is known that a state of acute anxiety stimulates the sympathetic nervous system (SNS) causing increased muscle tension, increased nociceptive input and increased sensitivity to pain stimuli , thus explaining why anxiety may correspond to increased pain perception. | *It is known that a state of acute anxiety stimulates the sympathetic nervous system (SNS), causing increased muscle tension, increased nociceptive input and increased sensitivity to pain stimuli, thus explaining why anxiety may correspond to increased pain perception. | ||
*Corticosteroid hormones, for example, released after stress may play a part in pain modulation<ref>Dunne FJ and Dunne CA. Fibromyalgia, psychiatric co-morbidity, and the somato-sensory cortex. British Journal of Medical Practitioners; 5, 2 (2012): 522-525</ref> as cortisol (a steroid hormone known to stimulate the SNS) has been found in higher levels in those with chronic pain<ref>Vachon-Presseau E, Roy M, Martel M, Caron E, Marin M, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ and Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain Journal; 136 (2013), DOI: http://dx.doi.org/10.1093/brain/aws371. Accessed 10/11/14. fckLR[</ref>. | *Corticosteroid hormones, for example, released after stress may play a part in pain modulation<ref name=":3">Dunne FJ and Dunne CA. Fibromyalgia, psychiatric co-morbidity, and the somato-sensory cortex. British Journal of Medical Practitioners; 5, 2 (2012): 522-525</ref>, as cortisol (a steroid hormone known to stimulate the SNS) has been found in higher levels in those with chronic pain<ref>Vachon-Presseau E, Roy M, Martel M, Caron E, Marin M, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ and Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain Journal; 136 (2013), DOI: http://dx.doi.org/10.1093/brain/aws371. Accessed 10/11/14. fckLR[</ref>. | ||
*Furthermore neurotransmitters, neuropeptides and pro-inflammatory cytokines have been found to either mediate or modulate pain. Therefore these physiological processes overlap in both anxiety and pain. | *Furthermore neurotransmitters, neuropeptides and pro-inflammatory cytokines have been found to either mediate or modulate pain. Therefore, these physiological processes overlap in both anxiety and pain. | ||
*There is evidence accumulating to show atypical sensory processing in the brain and dysfunction of skeletal muscle nociception, however the latter may not explain pain persistence in non-MSK pathologies where chronic pain has clearly been observed. | *There is evidence accumulating to show atypical sensory processing in the brain and dysfunction of skeletal muscle nociception<ref name=":3" /><ref>Gur A, Oktayoglu P. Central Nervous System Abnormalities in Fibromyalgia and Chronic Fatigue Syndrome: New Concepts in Treatment. Current Pharmaceutical Design. 2008;14(13):1274-1294. </ref>, however the latter may not explain pain persistence in non-MSK pathologies where chronic pain has clearly been observed. | ||
*The “pain matrix” | *The “pain matrix”<ref>Tracey I, Mantyh P. The Cerebral Signature for Pain Perception and Its Modulation. Neuron. 2007;55(3):377-391. </ref>, responsible for modulation of pain signals in the central nervous system (CNS), shares elements with brain networks responsible for stress, identifying another connection to the SNS. Pain contributes to feelings of anxiety due to fear of the cause, and if this persists a state of hypervigilance and avoidance behaviours may develop as a “maladaptive” coping response. | ||
*A more permanent state of anxiety results in chronic muscle tension and anticipatory anxiety which leads to | *A more permanent state of anxiety results in chronic muscle tension and anticipatory anxiety which leads to further disability. | ||
*Decreases in activities, especially those which provide meaning and reinforcement, may result in greater social isolation, decreased self-efficacy, increased feelings of uselessness and subsequent increases in anxiety and depression symptoms.<br> | *Decreases in activities, especially those which provide meaning and reinforcement, may result in greater social isolation, decreased self-efficacy, increased feelings of uselessness and subsequent increases in anxiety and depression symptoms.<br> | ||
This theory is closely interlinked with the ‘fear-avoidance’ model, a cycle prospected to play a vital part in sustenance of pain behaviours and has been shown to be an independent predictor of pain severity and disability among those with MSK pain.<br> | This theory is closely interlinked with the ‘fear-avoidance’ model, a cycle prospected to play a vital part in sustenance of pain behaviours and which has been shown to be an independent predictor of pain severity and disability among those with MSK pain<ref>Gatchel R, Neblett R, Kishino N, Ray C. Fear-Avoidance Beliefs and Chronic Pain. Journal of Orthopaedic & Sports Physical Therapy. 2016;46(2):38-43. </ref>.<br> | ||
Functional magnetic resonance imaging (fMRI) studies along with blood-oxygenated level dependent contrast (BOLD) techniques have been utilised in various studies to map common areas of brain activation by detecting changes in blood flow. These studies have been able to map similar areas of brain activation for anxiety and pain. It has been shown that there is exaggerated brain response in patients with social anxiety disorder<ref>Goldin PR and Gross JJ. Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder. Emotion; 10, 1 (2010): 83-91</ref> | Functional magnetic resonance imaging (fMRI) studies along with blood-oxygenated level dependent contrast (BOLD) techniques have been utilised in various studies to map common areas of brain activation by detecting changes in blood flow. These studies have been able to map similar areas of brain activation for anxiety and pain. It has been shown that there is exaggerated brain response in patients with social anxiety disorder<ref name=":0">Goldin PR and Gross JJ. Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder. Emotion; 10, 1 (2010): 83-91</ref>. | ||
*In one study these methods were used to identify highlighted areas in the brain when anxious patients reacted to negative self-beliefs. | *In one study these methods were used to identify highlighted areas in the brain when anxious patients reacted to negative self-beliefs. | ||
*The areas identified were the midline cortical regions such as | *The areas identified were the midline cortical regions such as: the ventromedial pre-frontal cortex (PFC), dorsomedial PFC, posterior cingulate cortex (implicated in self-referential process), emotional centre (amygdala) and memory area (hippocampal gyrus)<ref name=":0" />. | ||
*This indicates that there are integrated neural pathways associated with anxiety, pain processing, memory and concept of the self – the latter potentially implicating personality traits into anxiety and pain. <br> | *This indicates that there are integrated neural pathways associated with anxiety, pain processing, memory and concept of the self – the latter potentially implicating personality traits into anxiety and pain. <br> | ||
=== Depression === | |||
There is less known evidence about the physiology of depression compared to anxiety, however it has been postulated that people with depression or a depressive personality have a greater sensitivity to acute and chronic pain<ref name=":2" />. | |||
*Similarly to anxiety, excessive sympathetic activity and elevated pro-inflammatory cytokine production is also postulated in the | *Similarly to anxiety, excessive sympathetic activity and elevated pro-inflammatory cytokine production is also postulated in the etiology of depression, thus providing a potential physiological link between all three conditions and a possible reason for the overlapping prevalence within patient presentations. | ||
*fMRI studies have been conducted in patients with depressive disorders and similar findings have been identified as in anxious patients | *fMRI studies have been conducted in patients with depressive disorders and similar findings have been identified as in anxious patients. | ||
*In diabetic patients with depression, similar brain activation was identified in both neuropathic and non-neuropathic pain patients; these were PFC, thalamus, insula and anterior cingulate cortex. | *In diabetic patients with depression, similar brain activation was identified in both neuropathic and non-neuropathic pain patients; these were PFC, thalamus, insula and anterior cingulate cortex. | ||
*Despite these CNS observations | *Despite these CNS observations, it is known that in healthy, non-depressed human volunteers, pain-intensity related haemodynamic changes have been observed in all of the above brain sites<ref>Porro CA. Functional imaging and pain: behavior, perception, and modulation. Neuroscientist; 9, 5 (2003): 354-369</ref>. Further studies compared the response of brain areas with BOLD techniques: | ||
*13 patients suffering from an acute episode of major depressive disorder (MDD) were investigated during painful stimulus application and compared to 13 control subjects<ref>Bär K, Wagner G, Koschke M, Boettger S, Boettger MK, Schlösser R and Sauer H. Increased prefrontal activation during pain perception in major depression. Biological Psychiatry; 62, 11 (2007): 1281-1287</ref>. | **13 patients suffering from an acute episode of major depressive disorder (MDD) were investigated during painful stimulus application and compared to 13 control subjects<ref>Bär K, Wagner G, Koschke M, Boettger S, Boettger MK, Schlösser R and Sauer H. Increased prefrontal activation during pain perception in major depression. Biological Psychiatry; 62, 11 (2007): 1281-1287</ref>. | ||
*The results demonstrated increased activation of the pain matrix and increased thermal pain thresholds compared to healthy subjects. | **The results demonstrated increased activation of the pain matrix and increased thermal pain thresholds compared to healthy subjects. | ||
*They speculated that the brain area activation may be linked to an underlying prefrontal psychopathology in depression. | **They speculated that the brain area activation may be linked to an underlying prefrontal psychopathology in depression. | ||
In another BOLD study<ref>Strigo IA, Simmons AN, Matthews SC, Craig AD and Paulus MP. Major depressive disorder is associated with altered functional brain response during anticipation and processing of heat pain. Archives of General Psychiatry; 65, 11 (2008): 1275-1284</ref> patients with diagnosed MDD showed greater activation of pain-related brain sites (right insular, dorsal anterior cingulate, right amygdala) during anticipation of painful, heat stimuli compared with healthy subjects. | In another BOLD study<ref>Strigo IA, Simmons AN, Matthews SC, Craig AD and Paulus MP. Major depressive disorder is associated with altered functional brain response during anticipation and processing of heat pain. Archives of General Psychiatry; 65, 11 (2008): 1275-1284</ref> patients with diagnosed MDD showed greater activation of pain-related brain sites (right insular, dorsal anterior cingulate, right amygdala) during anticipation of painful, heat stimuli compared with healthy subjects. | ||
*Furthermore in MDD subjects, greater activation of the amygdala was associated with greater levels of perceived helplessness. | *Furthermore, in MDD subjects, greater activation of the amygdala was associated with greater levels of perceived helplessness. | ||
*This implies anticipation of pain may further pain experience and activation of the pain matrix potentially contributing to persistence of pain.<br> | *This implies anticipation of pain may further pain experience and activation of the pain matrix potentially contributing to persistence of pain.<br> | ||
In other studies the cingulate and PFC | In other studies, the cingulate and PFC have also been implicated in pain modulation and may contribute to chronic pain associated with fibromyalgia syndrome<ref name=":3" />. The brain is perceived as a mediator for nociceptive pain, but also for pain behaviours which are known to influence pain itself, likely causing its persistence. ‘Central sensitisation syndrome’ (CSS) is a highly recognised symptom of chronic pain syndrome. Hyperaesthesia and allodynia (increased sensitivity to painful and non-painful stimuli) are features of CSS and have been suggested to be mediated by synaptic strengthening and neuroplastic change at multiple CNS levels, further strengthening the hypothesis of the brain's role in nociceptive changes in the periphery<ref>Jensen T, Finnerup N. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. The Lancet Neurology. 2014;13(9):924-935. </ref>. | ||
*Other researchers have proposed that anxiety and depression may have similar physiological effects on programming in the brain providing more evidence for the relationship<ref name=":2" />. It is clear from research studies that physiology of depression, anxiety and pain are overlapping. However, it is difficult to differentiate the primary versus secondary cause.<span id="Catastrophising"></span> | |||
== Catastrophising == | |||
Pain catastrophizing refers to a negative cognitive-affective response to anticipated or actual pain<ref>Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758</ref>. It was formally introduced by Albert Ellis and was used to describe a maladaptive cognitive style in those with anxiety and depressive disorders<ref>Ellis A. Reason and Emotion in Psychotherapy. Lyle Stewart; NY, USA: 1962</ref>. The research focused on the fact that catastrophizing was an exaggerated and negative cognitive and emotional response during an actual or anticipated painful stimulation. Catastrophizing is often characterised by people magnifying their feelings about painful situations and ruminating about them, which can combine with feelings of helplessness<ref>Sullivan MJ, Rodgers WM, Kirsh I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain, 2001; 91: 147-154</ref>. Catastrophizing plays an important role in models of pain chronicity, showing a high correlation with both pain intensity and disability<ref>Meyer K, Tschopp A, Sprott H, Mannion AF. Association between catastrophizing and self-rated pain and disability in patients with chronic low back pain. Journal of Rehabilitation Medicine 2009; 41: 620-625</ref>. However, it is also apparent that fear-avoidance and depression are important predictors of pain intensity and disability<ref>Vlaeyen JW, Linton SJ. Fear avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000; 85: 317-332</ref>. High correlations between fear-avoidance and pain catastrophizing have been found<ref>George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: an experimental investigation in pain free individuals. European Journal of Pain 2006 July; 10 (5): 457-465</ref>. In studies, however, only pain catastrophizing predicted pain intensity<ref>George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: an experimental investigation in pain free individuals. European Journal of Pain 2006 July; 10 (5): 457-465</ref>. Additionally, catastrophizing has also been linked to adverse pain outcomes<ref>Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Pshycol Assess 1995; 7 (4): 524-532</ref><ref>Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Measures of clinical pain severity, disability and depression. Journal of Pain 2004 May; 5 (4): 195-211</ref><ref>Sullivan MJ, Thorn B, Haythornthwaite JA, Keele F, Martin M, Bradley LA, Lefebvere JC. Review: Theoretical perspectives on the relation between catastrophizing and pain. Clinical Journal of Pain 2001 March; 17 (1): 52-64</ref>. It can therefore be surmised that a reduction in pain catastrophizing will lead to a reduction in pain and disability<ref>Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain 2005; 113: 310-315</ref>. This highlights the importance of a multifactorial approach to pain management and the significance catastrophizing has in moulding the pain experience. | |||
== | === How Can We Assess the Levels of Catastrophizing? === | ||
Research has developed self-report instruments that can be used in various populations<ref>George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: an experimental investigation in pain free individuals. European Journal of Pain 2006 July; 10 (5): 457-465</ref>. Most commonly used is a 3-factor hierarchical structure for pain catastrophizing called [[Pain Catastrophizing Scale|The Pain Catastrophizing Scale]] (PCS). This scale incorporates magnification, rumination and helplessness. Highlighting the need for a multifactorial approach, participants are asked to rate the extent to which they experience each item by recalling previous experiences with pain<ref>Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758</ref>. More negative experiences with pain can correlate with higher levels of pain intensity and disability<ref>Meyer K, Tschopp A, Sprott H, Mannion AF. Association between catastrophizing and self-rated pain and disability in patients with chronic low back pain. Journal of Rehabilitation Medicine 2009; 41: 620-625</ref>. | |||
'''Why does this happen?''' | |||
There are various theoretical mechanisms of action including the appraisal theory<ref>Lazarus R, Folkman S. Stress, Appraisal and Coping. Springer; NY, USA: 1984</ref>, where the levels of helplessness the person is feeling will affect their ability to cope. Additional mechanisms include attention bias/information processing<ref>Eccleston C, Crombez G. Review: Pain demands attention. A Cognitive-affective model of the interruptive function of pain. Psychology Bulletin 1999 May; 125 (3): 356-366</ref>, where the catastrophizing amplifies the experience of pain via amplified attention biases to sensory and affective pain information<ref>Eccleston C, Crombez G. Review: Pain demands attention. A Cognitive-affective model of the interruptive function of pain. Psychology Bulletin 1999 May; 125 (3): 356-366</ref>, and an inability to suppress pain-related cognitions<ref>Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758</ref>. Evidence has also suggested that there is an increased nociceptive transmission via spinal gating mechanisms and a central sensitisation of pain. This may represent a central nervous mechanism which is contributing to the development, maintenance and aggravation of persistent pain<ref>Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758</ref><ref>Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp. Brain. Res. 2008 March; 186 (1): 79-85</ref><ref>Sullivan MJL, Rodgers WM, Wilson PM, Bell GJ, Murray TC, Fraser SN. An experimental investigation of the relation between catastrophizing and activity intolerance. Pain 2002; 100: 47-53</ref>. | |||
It is therefore apparent that both psychological and physiological factors play a major role in the perception, maintenance, experience and management of pain; and both directly influence each other. It is important to recognise these factors in both the acute and chronic pain patient in order to understand what aspects of their pain are a barrier to recovery. | |||
[[File:Figure 1 The Stress Response.png|thumb]] | |||
== Chronic Stress and Pain: Physiological Effects == | |||
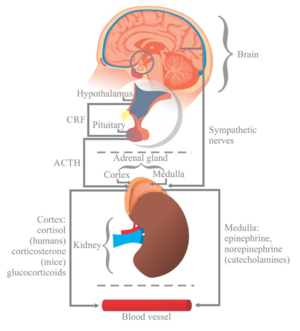
As discussed previously, psycho-social factors including depression, anxiety or fear-avoidance all factor into the pain experience and have distinct [[Chronic Pain and the Brain|acute and chronic effects on the brain]]. Additionally, chronic stress contributes to pain sensitisation via physiological changes occurring during the sympathetic response<ref name=":4">Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014 Dec;94(12):1816-25. doi: 10.2522/ptj.20130597. Epub 2014 Jul 17.</ref>. These changes are mediated primarily by cortisol, an anti-inflammatory neuroendocrine hormone. In the short term, the stress response is a physiologic flight-or-fight response, in which secretion of epinephirine and norepinephrine (sympathetic neurotransmitters) and cortisol promote survival. The immediate pro-inflammatory stage of the stress response, governed by the sympathetic neurotransmitters, is marked by increased heart rate, blood pressure, and respiration, as well as vasoconstriction and pupilary dilation. The latter stage of the stress response occurs as a delayed, systematic increase in cortisol levels 15 minutes after the initial stress response. The anti-inflammatory activity of cortisol acts to suppress non-vital organs, mobilise glucose reserves, and lower inflammation<ref name=":4" />. | |||
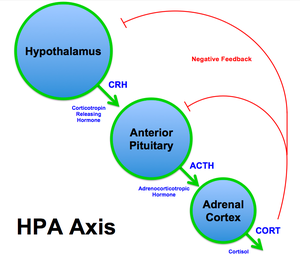
=== The Hypothalamic-Pituitary-Adrenal (HPA) Axis and Cortisol Dysfunction === | |||
[[File:HPA Axis Diagram (Brian M Sweis 2012).png|thumb]] | |||
At the onset of a stressful event, the amygdala signals release of corticotropin-releasing hormone (CRH) from the hypothalamus. The downstream effect of CRH subsequently triggers the release of adrenocorticotropic hormone (ACTH) via the anterior pituitary gland, which signals the release of cortisol from the adrenal cortex. This pathway, from hypothalamus to adrenal cortex, via CRH and ACTH signalling the release of cortisol, is referred to as the '''Hypothalamic-Pituitary-Adrenal (HPA) Axis'''<ref>Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383-95. </ref>. Although short-term activation of the HPA axis and cortisol release is anti-inflammatory and adaptive, prolonged and repeated stress exposure may lead to HPA axis exhaustion and cortisol dysfunction, including depletion, downregulation, and impairment of the negative feedback system<ref name=":4" />. Catastrophising and other maladaptive pain beliefs may similarly lead to reactivation of the stress response, prolonged cortisol secretion and dysfunction. These changes ultimately result in reduced cortisol efficacy in the secondary, anti-inflammatory, stage of the stress response. | |||
=== Cortisol Dysfunction: Effects on Pain === | |||
Cortisol dysfunction has been implicated in various inflammatory and autoimmune diseases, ranging from osteoporosis, rheumatoid arthritis, and chronic fatigue syndrome, among others<ref name=":4" />. HPA axis exhaustion and cortisol dysfunction can manifest as stress-induced hypocortisolism and are associated with musculoskeletal pain conditions including chronic low back pain and fibromyalgia. Additional studies have suggested hypocortisolism as a predictive risk factor for musculoskeletal pain<ref>Tak L, Rosmalen J. Dysfunction of stress responsive systems as a risk factor for functional somatic syndromes. Journal of Psychosomatic Research. 2010;68(5):461-468. </ref>. | |||
Therefore, it is critical to address both pain and non-pain-related stress in treating pain. Such measures may include objective cortisol readings in a medical setting, or subjective self-reports of stress as part of a screening or physiotherapy evaluation. By identifying stress factors or maladaptive coping strategies early in the screening process, [[Chronic Pain|providers can educate patients about chronic pain]] and the interplay between psychosocial factors and pain. Physiotherapists may also utilise [[Pain Neuroscience Education (PNE)|pain neuroscience education]]. Stress management, [[Psychological Approaches to Pain Management|psychological approaches to pain management]] and pain education can ultimately empower patients in their recovery as part of a multi-factorial pain rehabilitation program<ref name=":4" />. | |||
== References == | == References == | ||
| Line 144: | Line 160: | ||
<references /> | <references /> | ||
[[Category:Pain]] [[Category: | [[Category:PPA_Project]] | ||
[[Category:Pain]] | |||
[[Category:Pain Screening Tools]] | |||
[[Category:Mental Health]] | |||
[[Category:Older People/Geriatrics]] | |||
[[Category:Conditions]] | |||
[[Category:Interventions]] | |||
[[Category:Older People/Geriatrics - Conditions]] | |||
[[Category:Older People/Geriatrics - Interventions]] | |||
[[Category:Mental Health - Interventions]] | |||
Latest revision as of 12:30, 17 October 2023
Original Editor - Sharine Balicanta and Sarah Appleby
Top Contributors - Sharine Balicanta, Richard Benes, Kim Jackson, Jo Etherton, Tarina van der Stockt, Shaimaa Eldib, Simisola Ajeyalemi, Lauren Lopez, Admin, George Prudden, Kai A. Sigel, Luciana Segantin Lopes, Jess Bell, 127.0.0.1, Rachael Lowe and Evan Thomas
Introduction[edit | edit source]
The International Association for the Study of Pain (IASP) definition of pain highlights the multidimensional components of pain. The interaction of these dimensions (sensory-discriminative: intensity, location, quality and behaviour of pain; cognitive-evaluative: thoughts of the pain as influenced by previous experiences and knowledge; and motivational-affective: emotional responses like anger, anxiety and fear that motivate the response to pain)[1] all contribute to the complexity of the painful experience. An individual’s belief systems, pain understanding, thoughts and emotions: anxiety; depression; catastrophizing (or their psychology) influence how the brain interprets a noxious stimulus in relation to the meaning and level of threat the stimulus pose to one's well-being, and thereby influence the resulting output from the brain[2]. Such resulting changes in behaviour (i.e. avoidance)[3][4] such as fear avoidance, catastrophizing, pain-related anxiety, stress and learned helplessness can impact upon a pain experience and a patient's coping strategies. While this is unlikely to be an exclusive list of influential psychosocial factors, the following components are well researched and evidenced. This page will review each of these in turn.
Fear Avoidance[edit | edit source]
Pain and Fear: Fear is a typical emotional response to an experience that is perceived to be threatening, such as one that leads to a pain sensation[5][6]. Future avoidance of the painful activity is often the resulting behaviour adopted to protect oneself from a repeat of the fearful emotion and painful experience. This pattern can lead to fear and avoidance of work-related activities, movement, and re-injury[7]. Kinesophobia is a term used interchangeably with fear avoidance in health and pain literature. It is defined as “an excessive, irrational, and debilitating fear of physical movement and activity resulting from a feeling of vulnerability to painful injury or reinjury[8].”
Impact of Fear Avoidance on the Pain Experience[edit | edit source]
In response to acute tissue damage/injury, feelings of fear are heightened as part of a normal consequence of pain[4]. The individual will rest and protect the painful area as an adaptive behaviour to allow tissue healing to occur[4]. As the acute phase and initial tissue healing resolves, it has been suggested that some individuals can confront their fearful emotions and are able to resume normal activities, which ultimately helps extinguish their fears as they experience positive increase in mobility/movement unassociated with further increases in pain[5]. For others, they may be unable to overcome the fearful emotion and the resulting avoidance behaviour persists[7]. A cycle of continued activity avoidance and fearful emotions may ensue, resulting in chronic pain that may have an adverse effect on the musculoskeletal and cardiovascular system (disuse syndrome)[9], which fundamentally increases disability[4][7][10].
Several models have been proposed to explain the persistence of 'fear-avoidance' and its impact on pain and behaviour.
In the fear-avoidance model of pain, individuals who are likely to demonstrate ongoing fear-avoidance behaviours will be inclined to catastrophize[6][7]:
Vlaeyen and Linton[7] have described the interconnections contributing to fear avoidance as follows: with negative appraisals of a painful experience, fear avoidance behaviours are elicited. Due to hypervigilance, the avoidance behaviour begins to occur in anticipation rather than in response to pain. As there are fewer and fewer opportunities to correct the wrongful association of pain with the avoided activity, the behaviour perpetuates and fear avoidance persists.
While research has consistently shown an association between fear avoidance behaviour and pain-related fears[11][12][13][14][15] even when pain intensity and other variables were controlled in patients with chronic pain, there are conflicting studies about this association in patients with acute pain. Studies have shown that high levels of fear avoidance can be a predictor for future episodes of pain[16][17][18][19][20]. But on the contrary, studies[21] showed more support for the link between depression with poor function and disability at this early stage[22][23] In the learning pathway model, fear avoidance becomes a conditioned response through association of pain with movement[7][5][22]. The behaviour of avoiding expected pain-provoking movement or activities to prevent new episodes of pain is learned through experience[24]. The individual may anticipate similar experiences and associate non-painful or non-harmful experiences with pain, resulting in persistent fear-avoidance behaviour[7][22].
The following factors can affect the strength of association of pain with movement and the fear-avoidance behaviour that patients may exhibit:[25]
- existing beliefs about pain and the meaning of the symptoms
- beliefs about their own ability to control pain (self-efficacy)
- prior learning experiences (either direct experience or observational learning)
- expectations of recovery
- current emotional states (which include catastrophizing, anxiety, depression)
While the above models explain how fear avoidance becomes a maladaptive coping strategy and barrier to recovery in patients with chronic pain, this information may also be relevant to clinicians dealing with patients in the acute stages of injury. Any early fear-avoidance behaviours, especially for those who have strong negative emotions about their pain like anxiety or depression[12][22], could be identified. These patients could be monitored so they do not continue this coping strategy once the acute stage has passed. Knowing what factors affect fear-avoidance would help clinicians elicit these behaviours in their patients, expose any wrongful beliefs, attitudes or coping strategies (active vs passive) patient may have about their pain and recovery, and determine the best way to challenge them.
Physiotherapists managing these patients may find it useful to categorise the type of fear-avoidance patients exhibit. Rainville et al[5] proposed the following categories:
- Misinformed avoiders: have misinformed beliefs about movement and risk of damage or re-injury; may benefit from accurate information about pain and movement (pain physiology)
- Learned avoiders: learned the behaviour without being aware nor overly distressed about it; may benefit from gradual exposure to activity depending on pain tolerance
- Affective avoiders: are distressed and have strong negative cognitions about the pain; may need more cognition-based management and challenge beliefs
While not every patient with chronic pain will fall into any specific model or category, presenting an appropriate model may help the patient to realise the cycle of pain and fear-avoidance behaviour that individual has adopted. Furthermore, explaining how fear avoidance affects the physiological feelings they may be experiencing will help them to understand how their perception of pain may be altered[4][7][6]. For example:
- Increased physiological arousal/heightened muscle reactivity
- Associated anxiety and hyper-vigilance leads to increased attention to expected painful stimulus which in itself can intensify the pain
- Reduced participation in valued activities like work, leisure and social contacts can lead to mood changes like irritability, frustration and depression; these mood changes could further affect perception of pain
- Disuse or disability from persistent avoidance behavior will lead to pain with much less provocation than before (lower the threshold for experiencing future pain).
Several self-reported measures of fear avoidance could be used in the clinic to help assess fear avoidance. These include the following:
- Fear-Avoidance Beliefs Questionnaire (FABQ) identifies how beliefs about work and physical activity affect low back pain[26]
- Survey of Pain Attitudes (SOPA) assesses patients attitudes towards pain control, pain-related disability, medical cures of pain, solicitude of others, and medication for pain[27]
- Tampa Scale for Kinesiophobia (TSK) assesses fear of re-injury due to movement[8]
Anxiety and Depression[edit | edit source]
Anxiety[edit | edit source]
- Anxiety is a general term for several disorders that include uneasiness, apprehension, nervousness, fear and worrying.
- There is high prevalence of anxiety reported throughout the literature in patients with chronic pain syndromes of varying nature including the following: non-specified/widespread chronic pain[28][29][30][31][32][33], low back pain[34], traumatic musculoskeletal injury (TMsI)[35][36], rotator cuff tear [37]; and also in non-musculoskeletal (MSK) conditions such as phantom limb pain [38], prostatitis/chronic pelvic pain syndrome[39] and diabetic neuropathy[40].
- The prevalence of patients in each study purely with anxiety varied throughout, from 18 to 72% of those recruited. Unfortunately much of this literature does not solely focus on anxiety alone.
- The following have been commonly reported anxiety symptoms reported in patients with chronic pain: “not being able to stop worrying,” “worrying about too many different things” and “feeling as though something awful might happen.”
- A study conducted by Asmundson and Taylor in 1996 suggested the relation between anxiety sensitivity and developing pain-related fear and escape/avoidance in people with chronic pain[41].
A study[32] which aimed to evaluate the prediction of chronic pain looked at anxiety, depression and social stressors as risk factors and the severity of pain at 3, 6 and 12 months in 250 patients. They found a strong correlation between baseline anxiety which predicted pain severity at 12 months. Interestingly no correlation was found for social stressors or depression. In a TMsI study, anxiety was found to predict pain intensity at different time-frames post injury whereas depression, social support, length of hospital stay and self-efficacy had no substantial effect. They summarised that pain predicted anxiety and depression in the first year, but anxiety only predicted pain intensity from 12-24 months. Anxiety symptoms were therefore hypothesised as the primary causative factor of persistent pain in this cohort study. It is therefore important to address patients anxiety early to prevent it persisting and causing a negative barrier to their recovery.
One study examined the validity of a single question to screen for depression and anxiety[42].
- Single question screening tools were compared with validated questionnaires such as mini-international neuropsychiatric interview, Hospital Anxiety and Depression Scale (HADS) and Hopkins symptom checklist.
- It was found that the single questions demonstrated fair ability to detecting anxiety with a sensitivity of 68% and a good ability to exclude anxiety with a specificity of 85%.
- Therefore, single question screening tools are fairly effective in identifying anxiety and could be utilised into early assessments with chronic pain patients.
Depression[edit | edit source]
Depression is described as a general term for mental disorders which include: sadness, loss of interest or pleasure, feelings of low self-worth and low mood.
- Some frequently reported depressive symptoms among chronic pain patients are: “thinking of suicide/self harm” and “feeling down, depressed or hopeless”.
- Depression alone is associated with: chronic MSK[43] and widespread pain, knee pain[44], low back pain and chronic pelvic pain/prostatitis[45].
- In a study examining the impact of anxiety and depression in phantom limb pain (PLP) patients, mean depression scores were higher, although non-significant, in patients with non-PLP chronic pain syndromes[46].
A study of low back pain observed higher prevalence of depression symptoms (13.7%) compared to anxiety symptoms (9.5%)[47].
- Similarly, a cohort of 400 patients with chronic myofascial or neuropathic pain demonstrated higher prevalence of depression (93%) than anxiety (72%)[48].
Pain, Anxiety and Depression Anxiety and depression appear to be overlapping conditions. Much of the literature has acknowledged their joint effect in chronic pain patients[49].
A study in Brazil, which included 400 chronic pain patients, observed 54% with both of the psychiatric disorders compared to anxiety (18%) or depression (7%) alone[50]. In patients with chronic musculoskeletal pain, those with both anxiety and depression experienced the greatest pain severity, a highly significant finding. Furthermore combined psychiatric co-morbidity was strongly associated with disability days with the following: 18.1 days in pain-only patients, 32.2 in those with pain and anxiety, 38.0 days in those with pain and depression and 42.6 days in those with all three conditions. This study also found a significant correlation with reduced QoL when all three conditions presented together[33].
Thus it comes into question as to why these psychiatric disorders:
a) present together,
b) develop with the onset of traumatic pain,
c) appear to cause acute pain to persist; and
d) cause more severe functional deficit together than when presenting independently.
Many studies have looked into the pathophysiology of pain, anxiety and depression to find answers and to facilitate more successful treatment of these complex conditions[51][52][53].
Impact of Depression and Anxiety on the Pain Experience[edit | edit source]
Anxiety[edit | edit source]
A physiological model of anxiety may explain its role in pain perception;
- It is known that a state of acute anxiety stimulates the sympathetic nervous system (SNS), causing increased muscle tension, increased nociceptive input and increased sensitivity to pain stimuli, thus explaining why anxiety may correspond to increased pain perception.
- Corticosteroid hormones, for example, released after stress may play a part in pain modulation[54], as cortisol (a steroid hormone known to stimulate the SNS) has been found in higher levels in those with chronic pain[55].
- Furthermore neurotransmitters, neuropeptides and pro-inflammatory cytokines have been found to either mediate or modulate pain. Therefore, these physiological processes overlap in both anxiety and pain.
- There is evidence accumulating to show atypical sensory processing in the brain and dysfunction of skeletal muscle nociception[54][56], however the latter may not explain pain persistence in non-MSK pathologies where chronic pain has clearly been observed.
- The “pain matrix”[57], responsible for modulation of pain signals in the central nervous system (CNS), shares elements with brain networks responsible for stress, identifying another connection to the SNS. Pain contributes to feelings of anxiety due to fear of the cause, and if this persists a state of hypervigilance and avoidance behaviours may develop as a “maladaptive” coping response.
- A more permanent state of anxiety results in chronic muscle tension and anticipatory anxiety which leads to further disability.
- Decreases in activities, especially those which provide meaning and reinforcement, may result in greater social isolation, decreased self-efficacy, increased feelings of uselessness and subsequent increases in anxiety and depression symptoms.
This theory is closely interlinked with the ‘fear-avoidance’ model, a cycle prospected to play a vital part in sustenance of pain behaviours and which has been shown to be an independent predictor of pain severity and disability among those with MSK pain[58].
Functional magnetic resonance imaging (fMRI) studies along with blood-oxygenated level dependent contrast (BOLD) techniques have been utilised in various studies to map common areas of brain activation by detecting changes in blood flow. These studies have been able to map similar areas of brain activation for anxiety and pain. It has been shown that there is exaggerated brain response in patients with social anxiety disorder[59].
- In one study these methods were used to identify highlighted areas in the brain when anxious patients reacted to negative self-beliefs.
- The areas identified were the midline cortical regions such as: the ventromedial pre-frontal cortex (PFC), dorsomedial PFC, posterior cingulate cortex (implicated in self-referential process), emotional centre (amygdala) and memory area (hippocampal gyrus)[59].
- This indicates that there are integrated neural pathways associated with anxiety, pain processing, memory and concept of the self – the latter potentially implicating personality traits into anxiety and pain.
Depression[edit | edit source]
There is less known evidence about the physiology of depression compared to anxiety, however it has been postulated that people with depression or a depressive personality have a greater sensitivity to acute and chronic pain[33].
- Similarly to anxiety, excessive sympathetic activity and elevated pro-inflammatory cytokine production is also postulated in the etiology of depression, thus providing a potential physiological link between all three conditions and a possible reason for the overlapping prevalence within patient presentations.
- fMRI studies have been conducted in patients with depressive disorders and similar findings have been identified as in anxious patients.
- In diabetic patients with depression, similar brain activation was identified in both neuropathic and non-neuropathic pain patients; these were PFC, thalamus, insula and anterior cingulate cortex.
- Despite these CNS observations, it is known that in healthy, non-depressed human volunteers, pain-intensity related haemodynamic changes have been observed in all of the above brain sites[60]. Further studies compared the response of brain areas with BOLD techniques:
- 13 patients suffering from an acute episode of major depressive disorder (MDD) were investigated during painful stimulus application and compared to 13 control subjects[61].
- The results demonstrated increased activation of the pain matrix and increased thermal pain thresholds compared to healthy subjects.
- They speculated that the brain area activation may be linked to an underlying prefrontal psychopathology in depression.
In another BOLD study[62] patients with diagnosed MDD showed greater activation of pain-related brain sites (right insular, dorsal anterior cingulate, right amygdala) during anticipation of painful, heat stimuli compared with healthy subjects.
- Furthermore, in MDD subjects, greater activation of the amygdala was associated with greater levels of perceived helplessness.
- This implies anticipation of pain may further pain experience and activation of the pain matrix potentially contributing to persistence of pain.
In other studies, the cingulate and PFC have also been implicated in pain modulation and may contribute to chronic pain associated with fibromyalgia syndrome[54]. The brain is perceived as a mediator for nociceptive pain, but also for pain behaviours which are known to influence pain itself, likely causing its persistence. ‘Central sensitisation syndrome’ (CSS) is a highly recognised symptom of chronic pain syndrome. Hyperaesthesia and allodynia (increased sensitivity to painful and non-painful stimuli) are features of CSS and have been suggested to be mediated by synaptic strengthening and neuroplastic change at multiple CNS levels, further strengthening the hypothesis of the brain's role in nociceptive changes in the periphery[63].
- Other researchers have proposed that anxiety and depression may have similar physiological effects on programming in the brain providing more evidence for the relationship[33]. It is clear from research studies that physiology of depression, anxiety and pain are overlapping. However, it is difficult to differentiate the primary versus secondary cause.
Catastrophising[edit | edit source]
Pain catastrophizing refers to a negative cognitive-affective response to anticipated or actual pain[64]. It was formally introduced by Albert Ellis and was used to describe a maladaptive cognitive style in those with anxiety and depressive disorders[65]. The research focused on the fact that catastrophizing was an exaggerated and negative cognitive and emotional response during an actual or anticipated painful stimulation. Catastrophizing is often characterised by people magnifying their feelings about painful situations and ruminating about them, which can combine with feelings of helplessness[66]. Catastrophizing plays an important role in models of pain chronicity, showing a high correlation with both pain intensity and disability[67]. However, it is also apparent that fear-avoidance and depression are important predictors of pain intensity and disability[68]. High correlations between fear-avoidance and pain catastrophizing have been found[69]. In studies, however, only pain catastrophizing predicted pain intensity[70]. Additionally, catastrophizing has also been linked to adverse pain outcomes[71][72][73]. It can therefore be surmised that a reduction in pain catastrophizing will lead to a reduction in pain and disability[74]. This highlights the importance of a multifactorial approach to pain management and the significance catastrophizing has in moulding the pain experience.
How Can We Assess the Levels of Catastrophizing?[edit | edit source]
Research has developed self-report instruments that can be used in various populations[75]. Most commonly used is a 3-factor hierarchical structure for pain catastrophizing called The Pain Catastrophizing Scale (PCS). This scale incorporates magnification, rumination and helplessness. Highlighting the need for a multifactorial approach, participants are asked to rate the extent to which they experience each item by recalling previous experiences with pain[76]. More negative experiences with pain can correlate with higher levels of pain intensity and disability[77].
Why does this happen?
There are various theoretical mechanisms of action including the appraisal theory[78], where the levels of helplessness the person is feeling will affect their ability to cope. Additional mechanisms include attention bias/information processing[79], where the catastrophizing amplifies the experience of pain via amplified attention biases to sensory and affective pain information[80], and an inability to suppress pain-related cognitions[81]. Evidence has also suggested that there is an increased nociceptive transmission via spinal gating mechanisms and a central sensitisation of pain. This may represent a central nervous mechanism which is contributing to the development, maintenance and aggravation of persistent pain[82][83][84].
It is therefore apparent that both psychological and physiological factors play a major role in the perception, maintenance, experience and management of pain; and both directly influence each other. It is important to recognise these factors in both the acute and chronic pain patient in order to understand what aspects of their pain are a barrier to recovery.
Chronic Stress and Pain: Physiological Effects[edit | edit source]
As discussed previously, psycho-social factors including depression, anxiety or fear-avoidance all factor into the pain experience and have distinct acute and chronic effects on the brain. Additionally, chronic stress contributes to pain sensitisation via physiological changes occurring during the sympathetic response[85]. These changes are mediated primarily by cortisol, an anti-inflammatory neuroendocrine hormone. In the short term, the stress response is a physiologic flight-or-fight response, in which secretion of epinephirine and norepinephrine (sympathetic neurotransmitters) and cortisol promote survival. The immediate pro-inflammatory stage of the stress response, governed by the sympathetic neurotransmitters, is marked by increased heart rate, blood pressure, and respiration, as well as vasoconstriction and pupilary dilation. The latter stage of the stress response occurs as a delayed, systematic increase in cortisol levels 15 minutes after the initial stress response. The anti-inflammatory activity of cortisol acts to suppress non-vital organs, mobilise glucose reserves, and lower inflammation[85].
The Hypothalamic-Pituitary-Adrenal (HPA) Axis and Cortisol Dysfunction[edit | edit source]
At the onset of a stressful event, the amygdala signals release of corticotropin-releasing hormone (CRH) from the hypothalamus. The downstream effect of CRH subsequently triggers the release of adrenocorticotropic hormone (ACTH) via the anterior pituitary gland, which signals the release of cortisol from the adrenal cortex. This pathway, from hypothalamus to adrenal cortex, via CRH and ACTH signalling the release of cortisol, is referred to as the Hypothalamic-Pituitary-Adrenal (HPA) Axis[86]. Although short-term activation of the HPA axis and cortisol release is anti-inflammatory and adaptive, prolonged and repeated stress exposure may lead to HPA axis exhaustion and cortisol dysfunction, including depletion, downregulation, and impairment of the negative feedback system[85]. Catastrophising and other maladaptive pain beliefs may similarly lead to reactivation of the stress response, prolonged cortisol secretion and dysfunction. These changes ultimately result in reduced cortisol efficacy in the secondary, anti-inflammatory, stage of the stress response.
Cortisol Dysfunction: Effects on Pain[edit | edit source]
Cortisol dysfunction has been implicated in various inflammatory and autoimmune diseases, ranging from osteoporosis, rheumatoid arthritis, and chronic fatigue syndrome, among others[85]. HPA axis exhaustion and cortisol dysfunction can manifest as stress-induced hypocortisolism and are associated with musculoskeletal pain conditions including chronic low back pain and fibromyalgia. Additional studies have suggested hypocortisolism as a predictive risk factor for musculoskeletal pain[87].
Therefore, it is critical to address both pain and non-pain-related stress in treating pain. Such measures may include objective cortisol readings in a medical setting, or subjective self-reports of stress as part of a screening or physiotherapy evaluation. By identifying stress factors or maladaptive coping strategies early in the screening process, providers can educate patients about chronic pain and the interplay between psychosocial factors and pain. Physiotherapists may also utilise pain neuroscience education. Stress management, psychological approaches to pain management and pain education can ultimately empower patients in their recovery as part of a multi-factorial pain rehabilitation program[85].
References[edit | edit source]
- ↑ Gifford, LS.Butler, D.S. The Integration of Pain Sciences into Clinical Practice. Journal of Hand Therapy 1997; 10: 86-95
- ↑ Miller RM, Kaiser RS. Psychological characteristics of chronic pain: a review of current evidence and assessment tools to enhance treatment. Current pain and headache reports. 2018 Mar 1;22(3):22.
- ↑ Moseley GL. A pain neuromatrix approach to patients with chronic pain. Manual Therapy 2003 doi:10.1016/S1356-689X(03)00051-1
- ↑ 4.0 4.1 4.2 4.3 4.4 Turk D, Okifugi A. Psychological Factors in chronic pain: evolution and revolution. J of Consulting and Clinical Psychology 2002; 70: 678-690
- ↑ 5.0 5.1 5.2 5.3 Rainville J, Smeets RJEM, Bendix T, Tveito TH, Poiraudeau S, Indahl AJ. Fear-avoidance beliefs and pain avoidance in low back pain- translating research into clinical practice. The Spine Journal 2011; 11: 895-903
- ↑ 6.0 6.1 6.2 Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The Fear-Avoidance Model of Musculoskeletal Pain: Current State of Scientific Evidence. Journal of Behavioural Medicine 2007; 30: 77-94
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Vlaeyen JWS and Linton SJ. Fear –avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000; 85: 317-332
- ↑ 8.0 8.1 Kori, S.H., Miller, R.P., Todd, D.D. Kinesophobia: A new view of chronic pain. Pain Management 1990; 3:35-43
- ↑ Bortz, WM. The disuse syndrome: West J Med 1984; 41: 691-694
- ↑ Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000 Apr 1;85(3):317-32.
- ↑ Linton SJ. A Review of Psychological Risk Factors in Back and Neck pain. Spine 2000; 25: 1148-1156
- ↑ 12.0 12.1 Boersma K, Linton SJ. How does persistent pain develop? An analysis of the relationship between psychological variables, pain and function across stages of chronicity. Behav Res Ther 2005; 43: 1495-1507
- ↑ Boersma K, Linton SJ. Expectancy, fear and pain in the prediction of chronic pain and disability: A prospective analysis. Europ J of Pain 2006; 10: 551-557
- ↑ Grotle M, Vollestad NK, Veierod MB, Brox JI. Fear-avoidance beliefs and distress in relation to disability in acute and chronic back pain. Pain 2004; 112: 343-352.
- ↑ Picavet HS, Vlaeyen JWS, Schouten JS. Pain catastrophizing and kinesophobia: predictors of chronic back pain. Am J Epidemiol 2002; 156: 1028-1034
- ↑ Linton SJ, Buer N, Vlaeyen JWS, Hellsing, AL. Are fear –avoidance beliefs related to the inception of an episode of back pain? A prospective study. Psychol Helath 1999; 14: 1051-1059
- ↑ Klenerman ChM, Slade PD, Stanley IM, Pennie B, Reilly JP, Atchinsn LE, Troup JDG, Rose MJ. The Prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine 20; 4: 478-484
- ↑ Fritz JM, George SZ, Delitto A. The role of fear-avoidance beliefs in acute low back pain: relationships with current and future disability work status. Pain 2001; 94: 7-15
- ↑ Swinkels-Meewise IE, Roelofs J, Verbeek AL, Oostendorp RA, Vlaeyen JWS. Fear of movement/(re)injury, disability and participation in acute low back pain. Pain 2003; 105: 371-379
- ↑ Swinkels-Meewise IE, Roelofs J, Oostendorp RA, Verbeek AL, Vlaeyen JWS. Acute low back pain: pain-related fear and catastrophizing influence physical performance and perceived disability. Pain 2006; 120: 36-43
- ↑ Sieben JM, Portegijs PJM, Vlaeyen JWS, Knottnerus JA. Pain related fear at the start of a new low back pain episode. European J Pain 2005; 9: 635-641
- ↑ 22.0 22.1 22.2 22.3 Pincus T, Vogel S, Burton AK, Santos R, Field A. Fear avoidance and prognosis in back pain. A systematic review and synthesis of current evidence. Arthritis and Rheumatism 2006; 54: 3999-4010
- ↑ Boersma K, Linton SJ. How does persistent pain develop? An analysis of the relationship between psychological variables, pain and function across stages of chronicity. Behav Res Ther 2005; 43: 1495-1507
- ↑ Fordyce WE, Shelton JL, Dundore DE. The modification of avoidance learning in pain behaviors. J Behav Med 1982; 5: 405-414
- ↑ Turk DC, Monarch ES. Biopsychosocial perspective on chronic pain. Psychological approaches to pain management: A practitioner's handbook. 1996:3-2.
- ↑ Waddell G, Newton M, Henderson I, Somerville D, Main C. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance in chronic low back pain and disability. Pain 1993; 52: 157-168
- ↑ Jensen MP, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients' attitudes toward pain. J Psychosom Res 1987; 31: 393-400
- ↑ Valente MA, Ribeiro José, Jensen MP. Coping, Depression, Anxiety, Self-Efficacy and Social Support: Impact on Adjustment to Chronic Pain. Escritos de Psycologia; Vol 2 No.3 (2009): 8-17
- ↑ Tekur P, Chatametcha S, Hankey A, Negandra HR and Nagaranthna R. A comprehensive yoga programs improves pain, anxiety and depression in chronic low back pain patients more than exercise: An RCT. Complementary Medicines Therapy; Vol 20 Issue 3 (2012): 107-118
- ↑ Castro MMC and Daltro C. Sleep patterns and symptoms of anxiety and depression in patients with chronic pain. Arquivos de Neuropsiquiatria 67, 1 (2009): 25-28
- ↑ Castro MMC, Quarantini LC, Daltro C, Pires-Caldas M, Koenan KC, Kraychete DC and de Oliveira I. Comorbid depression and anxiety symptoms in chronic pain patients and their impact on health-related quality of life. Revista de Psyquiatria Clinica; 38, 4 (2011): 126-129
- ↑ 32.0 32.1 Bair MJ, Poleshuck EL, Wu J, Krebs EK, Damush TM, Tu W, Kroenke K. Anxiety but not social stressors predict 12-month depression and pain severity. Clin J Pain. 2013 Feb;29(2):95-101. doi: 10.1097/AJP.0b013e3182652ee9.
- ↑ 33.0 33.1 33.2 33.3 Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008 Oct;70(8):890-7. doi: 10.1097/PSY.0b013e318185c510..
- ↑ Bener A, Verjee M, Defeeah EE, Falah O, Al-Jushaishi T, Schlogl J, Sedeeq A and Khan S. Psychological factors: anxiety, depression, and somatization symptoms in low back pain patients. Journal of Pain Research; 6 (2013): 95-101
- ↑ Rosenbloom BN, Khan S, McCartney C and Katz J. Systematic review of persistent pain and psychological outcomes following traumatic musculoskeletal injury. Journal of Pain Research; 6 (2013): 39-51
- ↑ Castillo RC, Wegener ST, Heins SE, Haythornthwaite JA, MacKenzie EJ and Bosse MJ. Longitudinal relationships between anxiety, depression, and pain: Results from a two-year cohort study of lower extremity trauma patients. Pain; 154, 12 (2013): 2860-2866
- ↑ Potter MQ, Wylie JD, Greis PE, Burks RT and Tashjian RZ. Psychological Distress Negatively Affects Self-assessment of Shoulder Function in Patients With Rotator Cuff Tears. Clinical Orthopaedics and Related Research; 472, 12 (2014): 3926-3932
- ↑ Kazemi H, Ghassemi S, Fereshtehnejad SM, Amini A, Kolivand PH and Doroudi T. Anxiety and Depression in Patients with Amputated Limbs Suffering from Phantom Pain: A Comparative Study with Non-Phantom Chronic Pain. International Journal of Preventative Medicine; 4, 2 (2013): 218-225
- ↑ Ahn SG, Kim SH, Chung KI, Park KS, Cho SY and Kim HW. Depression, Anxiety, Stress Perception, and Coping Strategies in Korean Military Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Korean Journal of Urology; 53, 9 (2012): 643-648
- ↑ Jain R, Jain S, Raison CL and Maletic V, “Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators”, Current Diabetes Reports; 11, 4 (2011): 275-284
- ↑ Asmundson GJ, Taylor S. Role of anxiety sensitivity in pain-related fear and avoidance. Journal of behavioral medicine. 1996 Dec 1;19(6):577-86.
- ↑ Reme SE, Lie SA and Eriksen HR. Are 2 Questions Enough to Screen for Depression and Anxiety in Patients With Chronic Low Back Pain? Spine journal 39; 7 (2014) 455-462, doi:10.1097/BRS.0000000000000214, Accessed 06/11/14.
- ↑ Akkaya N, Atalay NŞ, Konukcu S and Şahin F. Effect of Number of Physical Therapy Sessions on Level of Pain, Depression, Anxiety, and Quality of Life. Journal Physical Medicine & Rehabilitation; 16 (2013): 8-13
- ↑ Phyomaung PP, Dubowitz J, Cicuttini FM, Fernando S, Wluka AE, Raaijmaakers P, Wang Y and Urquhart D. Are depression, anxiety and poor mental health risk factors for knee pain? A systematic review. BMC Musculoskeletal Disorders; 15, 10 (2014), doi:10.1186/1471-2474-15-10, Accessed 01/11/14.
- ↑ Tripp DA, Nickel JC and Katz L. A feasibility trial of a cognitive-behavioural symptom management program for chronic pelvic pain for men with refractory chronic prostatitis/chronic pelvic pain syndrome. Canadian Urological Association Journal; 5, 5 (2011), doi:10.5489/cuaj.10201, Accessed 05/11/14
- ↑ Elbinoune I, Amine B, Shyen S, Gueddari S, Abouqal R, Hajjaj-Hassouni N. Chronic neck pain and anxiety-depression: prevalence and associated risk factors. Pan Afr Med J. 2016 May 27;24:89. doi: 10.11604/pamj.2016.24.89.8831.
- ↑ Bener A, Verjee M, Dafeeah EE, Falah O, Al-Juhaishi T, Schlogl J, Sedeeq A, Khan S. Psychological factors: anxiety, depression, and somatization symptoms in low back pain patients. J Pain Res. 2013;6:95-101. doi: 10.2147/JPR.S40740.
- ↑ Castro Martha MC, Daltro C. Sleep patterns and symptoms of anxiety and depression in patients with chronic pain. Arq. Neuro-Psiquiatr. 2009 Mar;67(1): 25-28. Available from:https://doi.org/10.1590/S0004-282X2009000100007.
- ↑ McBeth J, McFarlane GJ, Hunt IM and Silman AJ. Risk factors for persistent chronic widespread pain: a community‐based study. Rheumatology; 40 (2001), doi: 10.1093/rheumatology/40.1.95, Accessed 10/11/14.
- ↑ Castro Martha MC, Quarantini Lucas C, Daltro C, Pires-Caldas M, Koenen KC, Kraychete DC, de Oliveira IR. Comorbid depression and anxiety symptoms in chronic pain patients and their impact on health-related quality of life. Rev. psiquiatr. clín. 2011;38(4):126-129. Available from:https://doi.org/10.1590/S0101-60832011000400002.
- ↑ Bair MJ, Robinson RL, Katon W and Kroenke K. Depression and Pain Comorbidity: A Literature Review. Arch Intern Med. 2003;163(20):2433–2445. doi:10.1001/archinte.163.20.2433
- ↑ Urits I, Burshtein A, Sharma M, Testa L, Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B and Kaye AD. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 2019;23(23). Available from: https://doi.org/10.1007/s11916-019-0757-1
- ↑ de Heer E, Gerrits M, Beekman A, Dekker J, van Marwijk H, de Waal M et al. The Association of Depression and Anxiety with Pain: A Study from NESDA. PLoS ONE. 2014;9(10):e106907.
- ↑ 54.0 54.1 54.2 Dunne FJ and Dunne CA. Fibromyalgia, psychiatric co-morbidity, and the somato-sensory cortex. British Journal of Medical Practitioners; 5, 2 (2012): 522-525
- ↑ Vachon-Presseau E, Roy M, Martel M, Caron E, Marin M, Chen J, Albouy G, Plante I, Sullivan MJ, Lupien SJ and Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain Journal; 136 (2013), DOI: http://dx.doi.org/10.1093/brain/aws371. Accessed 10/11/14. fckLR[
- ↑ Gur A, Oktayoglu P. Central Nervous System Abnormalities in Fibromyalgia and Chronic Fatigue Syndrome: New Concepts in Treatment. Current Pharmaceutical Design. 2008;14(13):1274-1294.
- ↑ Tracey I, Mantyh P. The Cerebral Signature for Pain Perception and Its Modulation. Neuron. 2007;55(3):377-391.
- ↑ Gatchel R, Neblett R, Kishino N, Ray C. Fear-Avoidance Beliefs and Chronic Pain. Journal of Orthopaedic & Sports Physical Therapy. 2016;46(2):38-43.
- ↑ 59.0 59.1 Goldin PR and Gross JJ. Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder. Emotion; 10, 1 (2010): 83-91
- ↑ Porro CA. Functional imaging and pain: behavior, perception, and modulation. Neuroscientist; 9, 5 (2003): 354-369
- ↑ Bär K, Wagner G, Koschke M, Boettger S, Boettger MK, Schlösser R and Sauer H. Increased prefrontal activation during pain perception in major depression. Biological Psychiatry; 62, 11 (2007): 1281-1287
- ↑ Strigo IA, Simmons AN, Matthews SC, Craig AD and Paulus MP. Major depressive disorder is associated with altered functional brain response during anticipation and processing of heat pain. Archives of General Psychiatry; 65, 11 (2008): 1275-1284
- ↑ Jensen T, Finnerup N. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. The Lancet Neurology. 2014;13(9):924-935.
- ↑ Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758
- ↑ Ellis A. Reason and Emotion in Psychotherapy. Lyle Stewart; NY, USA: 1962
- ↑ Sullivan MJ, Rodgers WM, Kirsh I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain, 2001; 91: 147-154
- ↑ Meyer K, Tschopp A, Sprott H, Mannion AF. Association between catastrophizing and self-rated pain and disability in patients with chronic low back pain. Journal of Rehabilitation Medicine 2009; 41: 620-625
- ↑ Vlaeyen JW, Linton SJ. Fear avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000; 85: 317-332
- ↑ George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: an experimental investigation in pain free individuals. European Journal of Pain 2006 July; 10 (5): 457-465
- ↑ George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: an experimental investigation in pain free individuals. European Journal of Pain 2006 July; 10 (5): 457-465
- ↑ Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Pshycol Assess 1995; 7 (4): 524-532
- ↑ Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Measures of clinical pain severity, disability and depression. Journal of Pain 2004 May; 5 (4): 195-211
- ↑ Sullivan MJ, Thorn B, Haythornthwaite JA, Keele F, Martin M, Bradley LA, Lefebvere JC. Review: Theoretical perspectives on the relation between catastrophizing and pain. Clinical Journal of Pain 2001 March; 17 (1): 52-64
- ↑ Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain 2005; 113: 310-315
- ↑ George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: an experimental investigation in pain free individuals. European Journal of Pain 2006 July; 10 (5): 457-465
- ↑ Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758
- ↑ Meyer K, Tschopp A, Sprott H, Mannion AF. Association between catastrophizing and self-rated pain and disability in patients with chronic low back pain. Journal of Rehabilitation Medicine 2009; 41: 620-625
- ↑ Lazarus R, Folkman S. Stress, Appraisal and Coping. Springer; NY, USA: 1984
- ↑ Eccleston C, Crombez G. Review: Pain demands attention. A Cognitive-affective model of the interruptive function of pain. Psychology Bulletin 1999 May; 125 (3): 356-366
- ↑ Eccleston C, Crombez G. Review: Pain demands attention. A Cognitive-affective model of the interruptive function of pain. Psychology Bulletin 1999 May; 125 (3): 356-366
- ↑ Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758
- ↑ Quartana PJ, Campbell CM, and Edwards RR. Pain Catastrophizing: a Critical Review. Expert Review of Neuropathics, May 2009; 9 (5): 745-758
- ↑ Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp. Brain. Res. 2008 March; 186 (1): 79-85
- ↑ Sullivan MJL, Rodgers WM, Wilson PM, Bell GJ, Murray TC, Fraser SN. An experimental investigation of the relation between catastrophizing and activity intolerance. Pain 2002; 100: 47-53
- ↑ 85.0 85.1 85.2 85.3 85.4 Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014 Dec;94(12):1816-25. doi: 10.2522/ptj.20130597. Epub 2014 Jul 17.
- ↑ Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383-95.
- ↑ Tak L, Rosmalen J. Dysfunction of stress responsive systems as a risk factor for functional somatic syndromes. Journal of Psychosomatic Research. 2010;68(5):461-468.