Neuropathic Pain: Difference between revisions
No edit summary |
(Added epidemiology) |
||
| (25 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<div class="editorbox"> | <div class="editorbox"> | ||
'''Original Editor '''- [[User:Kerstin McPherson|Kerstin McPherson]] | '''Original Editor '''- [[User:Kerstin McPherson|Kerstin McPherson]] | ||
| Line 20: | Line 18: | ||
== Aetiology == | == Aetiology == | ||
Conditions frequently associated with neuropathic pain can be categorised into two major groups: pain due to damage in the central nervous system (CNS) and pain due to damage to the peripheral nervous system (PNS). | Conditions frequently associated with neuropathic pain can be categorised into two major groups: pain due to damage in the central nervous system (CNS) and pain due to damage to the peripheral nervous system (PNS). | ||
[[File: | [[File:Anatomical_division_of_the_nervous_system.png|alt=Anatomical division of the nervous system|frameless|700x700px]] | ||
{| class="wikitable" | {| class="wikitable" | ||
|+'''Conditions associated with neuropathic pain''' | |+'''Conditions associated with neuropathic pain''' | ||
| Line 33: | Line 31: | ||
|- | |- | ||
|[[Syringomyelia]] and [[syringobulbia]] | |[[Syringomyelia]] and [[syringobulbia]] | ||
|Post-amputation stump and phantom limb pain | |Post-amputation stump and [[Phantom Limb Pain|phantom limb pain]] | ||
|- | |- | ||
|[[Trigeminal Neuralgia|Trigeminal]] and glossopharyngeal neuralgias | |[[Trigeminal Neuralgia|Trigeminal]] and glossopharyngeal neuralgias | ||
| Line 39: | Line 37: | ||
|- | |- | ||
|Neoplastic and other space-occupying lesions | |Neoplastic and other space-occupying lesions | ||
|Disease related neuropathies: Diabetic neuropathy, cancer-related neuropathies and HIV-related neuropathy | |Disease related neuropathies: [[Diabetic Neuropathy|Diabetic neuropathy]], [[Chemotherapy-Induced Peripheral Neuropathy (CIPN)|cancer-related neuropathies]] and HIV-related neuropathy | ||
|- | |- | ||
| | |Central pain in [[Multiple Sclerosis (MS)|Multiple Sclerosis]] | ||
|Complex regional pain syndrome (CRPS) type 2 | |[[Complex Regional Pain Syndrome (CRPS)|Complex regional pain syndrome]] (CRPS) type 2 | ||
|} | |} | ||
[[File:Neuropathic_pain_conditions.jpg|alt=Image indicating the pain patterns in different neuropathic pain conditions|1223x1223px]] | |||
Also see '''[[Neuropathies]]''' | Also see '''[[Neuropathies]]''' | ||
== Epidemiology == | == Epidemiology == | ||
The estimated prevalence of neuropathic pain is about 5%, but due to the difficulty in accurately diagnosing neuropathic pain, this number is likely an underestimation<ref name=":4">Bannister K, Sachau J, Baron R, Dickenson AH. [https://www.annualreviews.org/doi/10.1146/annurev-pharmtox-010818-021524 Neuropathic pain: mechanism-based therapeutics.] Annual Review of Pharmacology and Toxicology. 2020 Jan 6;60:257-74.</ref>. | The estimated prevalence of neuropathic pain is about 5%, but due to the difficulty in accurately diagnosing neuropathic pain, this number is likely an underestimation<ref name=":4">Bannister K, Sachau J, Baron R, Dickenson AH. [https://www.annualreviews.org/doi/10.1146/annurev-pharmtox-010818-021524 Neuropathic pain: mechanism-based therapeutics.] Annual Review of Pharmacology and Toxicology. 2020 Jan 6;60:257-74.</ref>. Neuropathic pain is not an inevitable consequence of a neural lesion, and the progression from acute to chronic neuropathic pain occurs only in a minority and is influenced by a huge number of factors<ref name=":5" />. | ||
The following '''risk factors''' could predispose an individual to the development of neuropathic pain<ref name=":4" />: | Below is a table presenting the prevalence of neuropathic pain (NP) in various conditions<ref>Di Stefano G, Di Lionardo A, Di Pietro G, Truini A. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7821938/ Neuropathic pain related to peripheral neuropathies according to the iasp grading system criteria.] Brain Sciences. 2020 Dec 22;11(1):1.</ref>: | ||
{| class="wikitable" | |||
|+ | |||
!'''Condition''' | |||
!'''Prevalence of NP''' | |||
|- | |||
|Diabetic Polyneuropathy | |||
|37-42% | |||
|- | |||
|Brachial Plexus Injury | |||
|56% | |||
|- | |||
|Postsurgical pain(thoracic/breast surgery) | |||
|up to 68% | |||
|- | |||
|Postsurgical pain (hip/knee arthroplasty) | |||
|6% | |||
|} | |||
The following '''risk factors''' could predispose an individual to the development of chronic neuropathic pain<ref name=":4" />: | |||
* Age 50-64 years | * Age 50-64 years | ||
| Line 58: | Line 76: | ||
== Pathophysiology == | == Pathophysiology == | ||
Neuropathic pain is the result of disease or lesion of the somatosensory nervous system which results in altered and disordered transmission of sensory signals<ref name=":4" />. Changes in nerve function occur both at the site of the injury and areas around the injury<ref name=":1" />. Peripheral, spinal and central changes result in | Neuropathic pain is the result of a disease or lesion of the somatosensory nervous system which results in altered and disordered transmission of sensory signals<ref name=":4" />. Changes in nerve function occur both at the site of the injury and areas around the injury<ref name=":1" />. Peripheral, spinal and central changes result in increased excitability and facilitation of signals, and a loss of inhibition<ref name=":4" />. With increasing chronicity, there is increased involvement of central changes. The pathophysiology is complex and still not fully understood. | ||
{| class="wikitable" | {| class="wikitable" | ||
|+Pathophysiology in Neuropathic pain<ref name=":4" /><ref>Ochoa J, Torebjork HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–854.</ref><ref>Finnerup NB, Kuner R, Jensen TS. [ | |+Pathophysiology in Neuropathic pain<ref name=":4" /><ref>Ochoa J, Torebjork HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–854.</ref><ref name=":5">Finnerup NB, Kuner R, Jensen TS. [https://journals.physiology.org/doi/full/10.1152/physrev.00045.2019?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org Neuropathic pain: from mechanisms to treatment.] Physiological reviews. 2020 Nov 3.</ref> | ||
! | ! | ||
!'''Peripheral changes''' | !'''Peripheral changes''' | ||
| Line 67: | Line 85: | ||
|- | |- | ||
|'''Spontaneous pain''' | |'''Spontaneous pain''' | ||
|Ectopic impulses along Aβ, Aδ, and C fibres of neuromas, | |Ectopic impulses along Aβ, Aδ, and C fibres of neuromas, nerve ends and nerve roots | ||
|Ectopic impulses at the dorsal root ganglia | |Ectopic impulses at the dorsal root ganglia | ||
|Ectopic impulses at the thalamus | |Ectopic impulses at the thalamus | ||
|- | |- | ||
|'''Sensitisation''' | |'''Sensitisation''' | ||
|Alterations in ion-channels: increased function of sodium channels leading to increased excitability | |Alterations in ion-channels: increased function of sodium channels leading to increased excitability; loss of potassium channels to modulate nerve activity | ||
|Increased expression and function of calcium channels leading to increased transmitter release and enhanced excitability of spinal neurons | |Increased expression and function of calcium channels leading to increased transmitter release and enhanced excitability of spinal neurons; expanded receptor fields through NMDA-receptor activation; A-fibre sprouting into laminae I and II of the dorsal horn, which can result in allodynia | ||
|Altered descending inhibition at the brain-stem (modulated by the cingulate cortex and amygdala); Limbic areas drive anxiety, depression and sleep problems; cortical reorganisation | |||
|Limbic areas drive anxiety, depression and sleep problems | |||
|} | |} | ||
=== Abnormal Impulse Generating Sites (AIGS) === | |||
* Abnormal Impulse Generating Sites (AIGS) are defined as the unmyelinated sites along the damaged axon in which the number, kind and excitability of ion channels are altered. This change in ion channel distribution make the site susceptible to non-noxious stimuli such as mechanical, chemical, or thermal stimuli<ref>Greening J, Lynn B. Minor peripheral nerve injuries an underestimated source of pain? Manual Therapy, 1998; 3: 187-194</ref><ref>Gifford L. Acute low cervical nerve root conditions: symptom presentation and pathobiological reasoning. Manual Therapy, 2001; 6: 106-115</ref>. | |||
* The high density of ion channels may result in a resting potential close to the threshold, leading to spontaneous ectopic impulses.<ref>Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion — a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38</ref> | |||
* AIGS fire antidromically and orthodromically, resulting in constant noxious stimuli into the central nervous system and [[Neurogenic Inflammation|neurogenic inflammation]] in the tissues. For this reason AIGS can persist even though the primary injury on the axon is completely healed. | |||
* Where axons are disconnected from their distal targets (eg. amputation), inflammation and sprouting occur, resulting in neuromas. A neuroma may become an anatomical site for generating nociceptive impulses. | |||
* Together, neuromas, AIGS and dorsal root ganglion changes in receptors can be the peripheral nervous system cause of[[Phantom Limb Pain|phantom limb pain.]]<ref>Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. ''J Clin Invest''. 2018;128(6):2168-2176.</ref> | |||
=== Central Sensitisation === | === Central Sensitisation === | ||
The term central sensitisation (CS) is often used | The term [[Central Sensitisation|central sensitisation]] (CS) is often incorrectly used synonymously with neuropathic pain. CS is however a mechanism that can occur independent of nerve lesions, but is often an underlying mechanism involved in chronic neuropathic pain. Central sensitisation is a state where spinal excitability increases due to repeated painful inputs<ref name=":5" />. CS results in decreased thresholds and increased response to nociceptive input. | ||
* Following a peripheral origin, central sensitisation may develop as a result of ectopic neuronal activity in the [[Spinal cord anatomy|spinal cord]] dorsal horn, implying a potential autonomous pain-generating mechanism.<ref name=":2">Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS . [https://pubmed.ncbi.nlm.nih.gov/24704366/ Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy.] Pain, 2014; 155 (7): 1272-9</ref> | * Following a peripheral origin, central sensitisation may develop as a result of ectopic neuronal activity in the [[Spinal cord anatomy|spinal cord]] dorsal horn, implying a potential autonomous pain-generating mechanism.<ref name=":2">Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS . [https://pubmed.ncbi.nlm.nih.gov/24704366/ Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy.] Pain, 2014; 155 (7): 1272-9</ref> This leads to an increase in the size of the sensory receptive field, a reduced threshold for nociception, and hypersensitivity to various innocuous stimuli. | ||
* First order [[Neurone|neuron]]<nowiki/>[[Neurone|s]] may increase their firing if they are partially damaged and increase the number of sodium channels. | |||
* Ectopic discharges are a result of enhanced depolarization at certain sites in the fibre, leading to spontaneous pain and movement-related pain. | |||
* First order [[Neurone|neuron]]<nowiki/>s may increase their firing if they are partially damaged and increase the number of sodium channels. | * Inhibitory circuits may be impaired at the level of the dorsal horn or [[Brainstem|brain stem]] (or both) allowing pain impulses to travel unopposed. See [[Pain Facilitation and Inhibition|'''Pain Facilitation and Inhibition''']] | ||
* Ectopic discharges are a result of enhanced depolarization at certain sites in the | * In addition, there may be alterations in the central processing of pain when. Second- and third-order neurons develop a “memory” of pain and become sensitised. There is then heightened sensitivity in spinal neurons and reduced activation thresholds. <ref name=":0" /> | ||
* Inhibitory | |||
* In addition, there may be alterations in the central processing of pain when | |||
== Clinical Presentation == | == Clinical Presentation == | ||
| Line 125: | Line 116: | ||
!'''Positive signs''' | !'''Positive signs''' | ||
!'''Negative signs''' | !'''Negative signs''' | ||
|- | |- | ||
|''Features'' | |''Features'' | ||
|'''Paraesthesia:''' crawling/tingling sensation | |'''Paraesthesia:''' crawling/tingling sensation | ||
'''Spontaneous pain:''' burning, shooting, electric-like sensations | '''Spontaneous pain:''' burning, shooting, electric-like sensations '''Hyperalgesia:''' increased sensitivity to noxious stimuli '''[[Allodynia]]:''' pain in response to non-noxious stimuli | ||
'''Hyperalgesia:''' increased sensitivity to noxious stimuli | |||
'''[[Allodynia]]:''' pain in response to non-noxious stimuli | |||
'''Summation:''' progressive worsening of pain with repetitive stimulation | '''Summation:''' progressive worsening of pain with repetitive stimulation | ||
|Hyposthesia: sensory loss and numbness | |'''Hyposthesia''': sensory loss and numbness | ||
Sensory loss is in the distribution of the damaged nerve or in the areas that correspond to the spinal/cortical region that has been damaged | Sensory loss is in the distribution of the damaged nerve or in the areas that correspond to the spinal/cortical region that has been damaged | ||
|} | |} | ||
Patients will often report spontaneous pain and sensations of ‘pins and needles,’ shooting, burning, stabbing, crawling, and paroxysmal pain (electric-shock like). These sensations affect not only the patient’s sensory system, but also the patient’s well-being, mood and focus. | '''History:''' Patients will often report spontaneous pain and sensations of ‘pins and needles,’ shooting, burning, stabbing, crawling, and paroxysmal pain (electric-shock like). These sensations affect not only the patient’s sensory system, but also the patient’s well-being, mood and focus. The onset of pain may be delayed, as is often the case in central post-stroke pain, or phantom limb pain where neuropathic symptoms may start months or years after the primary event of injury. | ||
'''Clinical findings:''' There may be decreased reflexes, weakness and autonomic changes<ref name=":5" />. Sensation testing will reveal abnormalities - if sensation is normal, it is highly likely that the pain experienced is of neuropathic origin. | |||
Neuropathic pain is often mixed with other pain mechanisms ([[Nociception|nociceptive]], [[Central Sensitisation|central sensitisation]]), which may result in a mixed clinical presentation. | Neuropathic pain is often mixed with other pain mechanisms ([[Nociception|nociceptive]], [[Central Sensitisation|central sensitisation]]), which may result in a mixed clinical presentation. | ||
== Assessment == | == Diagnosis and Assessment == | ||
One of the challenges | One of the challenges of neuropathic pain, is the ability to assess it. Assessing quality, intensity and improvement, as well as accurately diagnosing neuropathic pain is often complex. | ||
There are, however, some diagnostic tools that may assist clinicians in evaluating neuropathic pain. eg [[Electrodiagnosis|nerve conduction studies and sensory-evoked potentials]] can identify and quantify the extent of damage to sensory | There are, however, some diagnostic tools that may assist clinicians in evaluating neuropathic pain. eg [[Electrodiagnosis|nerve conduction studies and sensory-evoked potentials]] can identify and quantify the extent of damage to sensory (but not nociceptive) pathways by monitoring neurophysiological responses to electrical stimuli. | ||
It is also very important to perform a thorough [[Neurological Assessment|neurological]] evaluation to identify motor, sensory and autonomic dysfunctions. Use a paperclip/pin to test pinprick sensation, cotton to test light touch and a cooled reflex hammer to test cold sensation<ref name=":7">Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, Levy RM, Hunter CW. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6544553/ A comprehensive algorithm for management of neuropathic pain]. Pain Medicine. 2019 Jun 1;20(Supplement_1):S2-12.</ref>. | |||
{{#ev:youtube|Arukf5l2D0Y}} | |||
{| | |||
|+Grading System for Neuropathic Pain (NP) diagnosis <ref name=":5" /> | |||
! | |||
!'''Possible NP''' | |||
|'''Probable NP''' | |||
|'''Confirmed NP''' | |||
|- | |||
! | |||
!History of relevant neurological lesion or disease; pain distribution neuroanatomically plausible | |||
|Pain associated with sensory signs in the same neuroanatomically plausible distribution on clinical examination | |||
|Diagnostic test confirming a lesion or diseae of the somatosensory nervous system explaining the pain | |||
|- | |||
! | |||
!History and pain and sensory descriptors are compatible with a lesion in the nervous system and not an inflammatory or non-neurological condition | |||
|The area of sensory changes may extend beyond, be within, or overlap the area of pain | |||
|Requires that other types of pain are excluded or highly unlikely to entirely explain the pain condition | |||
|} | |||
=== Quantitative Sensory Testing === | === Quantitative Sensory Testing === | ||
QST is often time consuming and | [[Quantitative Sensory Testing (QST)|Quantitative Sensory Testing]] (QST) examines thermal and mechanical sensory function and can be used to quantify sensory loss or sensory gain. It can be particularly useful when basic sensory testing does not reveal any somatosensory losses, but neuropathic pain is still suspected. | ||
QST is often time consuming and involves expensive equipment, which limits is application in clinical practice. There are however simplified bedside QST tests that can be useful to gain improved understanding related underlying pain mechanisms<ref name=":4" />. | |||
=== Outcome Measures === | === Outcome Measures === | ||
The following tools can be used to screen for neuropathic pain and to monitor neuropathic pain over time | The following tools can be used to screen for neuropathic pain and to monitor neuropathic pain over time<ref name=":7" />: | ||
* [[DN4 questionnaire|DN4]] - a well validated screening tool to rank the probability that of peripheral or central neuropathic mechanisms are involved in chronic pain<ref name=":4" /> | * [[DN4 questionnaire|DN4]] - a well validated screening tool to rank the probability that of peripheral or central neuropathic mechanisms are involved in chronic pain<ref name=":4" /> | ||
* [[Leeds Assessment of Neuropathic Symptoms and Signs (LANSS)|LANSS]] | * [[Leeds Assessment of Neuropathic Symptoms and Signs (LANSS)|LANSS]] | ||
* [[PainDETECT]] | * [[PainDETECT]] | ||
The following outcome measures can be used to quantify the consequences of neuropathic pain: Pain Disability Index, Beck Depression Inventory, HADS, [[Pain Catastrophizing Scale|Pain Catastrophising Scale]], [[Brief Pain Inventory - Short Form|BPI]], [[EQ-5D]], [[36-Item Short Form Survey (SF-36)|SF-36]] | |||
== Management == | == Management == | ||
Neuropathic pain often responds poorly to standard pain treatments | Neuropathic pain often responds poorly to standard pain treatments. For some people, it can lead to significant disability. Since the cause of neuropathic pain can often not be treated, the focus of its management is on alleviating the symptoms which requires an multidisciplinary approach that combines therapies: | ||
# Medical management and the use of pharmacological agents | # Medical management and the use of pharmacological agents | ||
# Physiotherapy | # Physiotherapy | ||
# Low impact general physical activities | # Low impact general physical activities | ||
# | # Cognitive behavioural therapy<ref name=":8">Bernetti A, Agostini F, de Sire A, Mangone M, Tognolo L, Di Cesare A, Ruiu P, Paolucci T, Invernizzi M, Paoloni M. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7824970/ Neuropathic pain and rehabilitation: a systematic review of international guidelines.] Diagnostics. 2021 Jan 5;11(1):74.</ref> | ||
# Relaxation therapy | # Relaxation therapy | ||
# [[Massage|Massage therapy]] | # [[Massage|Massage therapy]] | ||
| Line 180: | Line 183: | ||
=== Pharmacological Management === | === Pharmacological Management === | ||
A number of pharmacological treatments can be used to manage neuropathic pain outside of specialist pain management services. However, there is considerable variation in how treatment is initiated, the dosages used | A number of pharmacological treatments can be used to manage neuropathic pain outside of specialist pain management services. However, there is considerable variation in how treatment is initiated, the dosages used, the order in which drugs are introduced, whether therapeutic doses are achieved and whether there is correct sequencing of therapeutic classes. A further issue is that a number of commonly used treatments are unlicensed for treating neuropathic pain, which may limit their use. These factors may lead to inadequate pain control, with considerable morbidity. The goal of pharmacotherapy is to selectively block the production and/or transmission of pain-producing afferent activity in the periphery, and to engage mechanisms of endogenous inhibition in the CNS<ref name=":6">Zusman M. Mechanisms of peripheral neuropathic pain: implications for musculoskeletal physiotherapy. Physical Therapy Reviews. 2008 Oct 1;13(5):313-23.</ref>. | ||
For commonly used pharmacological treatments see [[Neuropathic Pain Medication]] <ref name=":0" />. | For commonly used pharmacological treatments see [[Neuropathic Pain Medication]] <ref name=":0" />. See the summary table below. Note that trigeminal neuralgia presents an exception, where Carbamazepine is regarded as first-line treatment<ref name=":4" />. | ||
'''Recommendations from the Special Interest Group of Neuropathic Pain (NeuPSIG)'''<ref>Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6431761/ The neuropathic pain: An overview of the current treatment and future therapeutic approaches.] International Journal of Immunopathology and Pharmacology. 2019 Mar;33:2058738419838383.</ref> ''':''' | |||
{| class="wikitable" | |||
|+Pharmacotherapy for Peripheral Neuropathic pain | |||
!'''First line''' | |||
!'''Second line''' | |||
!'''Third line''' | |||
|- | |||
|Gabapentanoids (pregabalin, gabapentin) | |||
|Lidocaine patches | |||
|Strong opioids | |||
|- | |||
|Tricyclic antidepressants (TCAs) | |||
|Topical capsaicin | |||
|Botulinum toxin A | |||
|- | |||
|Selective serotonin-norepinephrine reuptake inhibitors (SNRIs) | |||
|Tramadol | |||
| | |||
|} | |||
It is very important to identify concomitant underlying pain mechanisms, as this could improve the effectiveness of pharmacological management by targeting additional mechanisms with different analgesic agents<ref name=":4" />. | It is very important to identify concomitant underlying pain mechanisms, as this could improve the effectiveness of pharmacological management by targeting additional mechanisms with different analgesic agents<ref name=":4" />. | ||
| Line 196: | Line 218: | ||
# '''Intrathecal treatments:''' In individuals with persistent pain that is severe and otherwise resistant to treatment, this offers a targeted drug delivery option to the painful part.<ref name=":3" /> | # '''Intrathecal treatments:''' In individuals with persistent pain that is severe and otherwise resistant to treatment, this offers a targeted drug delivery option to the painful part.<ref name=":3" /> | ||
=== | === Physiotherapy Management === | ||
Physiotherapy plays a pivotal role in the complex management of neuropathic pain and must be can considered as an alternative or complementary treatment to pharmacotherapy<ref name=":8" />.Physiotherapy aims to address [[Inflammation Acute and Chronic|inflammation]], stiffness, function and pain with exercise, mobilisation and electrotherapy modalities. Treatment aims to help the body heal itself by encouraging the production of the body's natural pain-relieving chemicals. | |||
<gallery widths="290" heights="200"> | |||
File:Neuro-HTtens-300x200.jpg|TENS therapy | |||
File:Laserfornp.jpg|Laser therapy | |||
File:Mirror therapy.jpg|Mirror therapy | |||
</gallery> | |||
[[ | # '''Acute pain relief:''' for patients who have severe pain, start with contrast baths, desensitisation techniques, and oedema control<ref name=":6" />. | ||
# '''Electrotherapy:''' [[Transcutaneous Electrical Nerve Stimulation (TENS)|TENS]] therapy has low quality evidence for being effective in the treatment of painful peripheral neuropathy<ref name=":8" /><ref>Gibson W, Wand BM, O'Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. 2017 Sep 14;9(9):CD011976</ref>. [[Low Level Laser Therapy|Low level laser therapy]] may provide pain relief for neuropathic pain <ref>de Andrade AL, Bossini PS, Parizotto NA. Use of low level laser therapy to control neuropathic pain: A systematic review. J Photochem Photobiol B. 2016 Nov;164:36-42. </ref>. | |||
# '''Therapeutic Exercise:''' Gentle ROM exercises and strengthening exercises can be useful to reorganise sprouting and cortical body representation, and to restore functional ROM and strength <ref>Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001 Dec;65(12):1378-82.</ref><ref>Trout KK. The neuromatrix theory of pain: implications for selected nonpharmacologic methods of pain relief for labor. J Midwifery Womens Health. 2004 Nov-Dec;49(6):482-8.</ref>. Studies have demonstrated the effectiveness of exercise in improving neuropathic pain in spinal cord injuries, stroke, multiple sclerosis, radiculopathy, diabetic polyneuropathy and HIV-related neuropathy <ref name=":9">Zhang YH, Hu HY, Xiong YC, Peng C, Hu L, Kong YZ, Wang YL, Guo JB, Bi S, Li TS, Ao LJ. E[https://www.frontiersin.org/articles/10.3389/fmed.2021.756940/full xercise for neuropathic pain: a systematic review and expert consensus.] Frontiers in Medicine. 2021 Nov 24;8:756940.</ref>. | |||
# '''Graded motor imagery:''' Mirror therapy and graded motor imagery are movement representation techniques and have been suggested to be beneficial in neuropathic pain management - especially for phantom limb pain , CRPS and stroke pain<ref>Kim SY, Kim YY. Mirror therapy for phantom limb pain. ''Korean J Pain''. 2012;25(4):272-274.</ref>. | |||
# '''Aerobic Exercise:''' Regular aerobic exercise has been shown to help manage chronic pain and can even help with nerve recovery<ref name=":9" />. There are studies showing that exercise may be an important part of the treatment and prevention of neuropathic pain after chemotherapy<ref>Majithia N, Loprinzi CL, Smith TJ.[https://pubmed.ncbi.nlm.nih.gov/27854104/ New Practical Approaches to Chemotherapy-Induced Neuropathic Pain: Prevention, Assessment, and Treatment.] Oncology (Williston Park). 2016 Nov 15;30(11)</ref>. Aerobic exercise resulted in improved mechanical allodynia and heat hyperalgesia in animal models of neuropathic pain <ref>Kami K, Tajima F, Senba E. [https://pubmed.ncbi.nlm.nih.gov/27484434/#:~:text=Physical%20exercise%2C%20such%20as%20forced,and%20spinal%20nerve%20ligation%20models. Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain.] Anat Sci Int. 2016 Aug 2. [Epub ahead of print]</ref>. The positive effects of exercise can be, at least in part, be explained by the stimulation of the endogenous opioid system resulting in exercise-induced hypoaglesia<ref name=":9" />. | |||
Nutrition is also an important factor when managing neuropathic pain. In this video, Dr. Andrea Furlan outlines 7 food groups to include if you have neuropathic pain, and 5 food groups to avoid. | |||
{{#ev:youtube|clbpGLcg_dM}}<ref>Dr. Andrea Furlan. #131 Seven Foods to improve NERVE PAIN and five foods and 5 to avoid if you have NEUROPATHIC pain. Available from: https://www.youtube.com/watch?v=clbpGLcg_dM&ab_channel=Dr.AndreaFurlan (accessed 17 September 2023). </ref> | |||
=== Multi-disciplinary Approach === | |||
All rehabilitation professionals and clinicians can play in important role in identifying and diagnosing neuropathic pain. The complex nature of neuropathic pain calls for an integrated team approach. Rehabilitation can often not occur effectively without adequate pharmacological pain management, emphasising the importance of a team approach. Psychosocial aspects should not be undermined, with a focus on improving participation and quality of life<ref name=":8" /> . Multi-disciplinary care of neuropathic pain has been shown to significantly decrease pain, improve function, mood and pain acceptance<ref name=":7" />. A patient-specific balance of pharmacological, psychological and physical management strategies need to be integrated. See the Physiopedia pages for each specific condition for more detailed integrated management approaches. | |||
'''A proposed comprehensive treatment algorithm for the management of neuropathic pain by Bates et al, 2019'''<ref name=":7" />''':''' | |||
[[File:Neuropathic Pain Algorithm.png|frameless|836x836px]] | |||
Please watch the following video for relevant infomration about neuropathic pain. | |||
Please watch the following video for relevant about neuropathic pain. | |||
Management of Neuropathic Pain<ref>Management of Neuropathic Pain. Available from: https://www.youtube.com/watch?v=hDu_WdRNDzo&t=796s</ref>{{#ev:youtube|hDu_WdRNDzo}} | Management of Neuropathic Pain<ref>Management of Neuropathic Pain. Available from: https://www.youtube.com/watch?v=hDu_WdRNDzo&t=796s</ref>{{#ev:youtube|hDu_WdRNDzo}} | ||
== Resources == | |||
'''[https://www.frontiersin.org/articles/10.3389/fmed.2021.756940/full Exercise Recommendations for specific neuropathic conditions]''' | |||
== References == | == References == | ||
<references /> | <references /> | ||
Latest revision as of 12:28, 20 December 2023
Original Editor - Kerstin McPherson
Top Contributors - Andeela Hafeez, Melissa Coetsee, Kerstin McPherson, Vanessa Rhule, Riccardo Ugrin, Lucinda hampton, Kim Jackson, Admin, Evan Thomas, Chrysolite Jyothi Kommu, Temitope Olowoyeye, Jo Etherton, WikiSysop, Vidya Acharya, Kapil Narale, Lauren Lopez, Wendy Walker, Carina Therese Magtibay, Oyemi Sillo, Amanda Ager and 127.0.0.1
Introduction[edit | edit source]
The International Association for the Study of Pain (2011) defines neuropathic pain as ‘pain caused by a lesion or disease of the somatosensory nervous system’. It is a complex, chronic pain state that presents a challenge to patients and clinicians.
It is the result of disease or damage anywhere along the neuraxis of the peripheral or central (spinal and supraspinal) nervous system[1].
- Central neuropathic pain is defined as ‘pain caused by a lesion or disease of the central somatosensory nervous system’.
- Peripheral neuropathic pain is defined as ‘pain caused by a lesion or disease of the peripheral somatosensory nervous system’.
Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies, symptoms, and underlying mechanisms[2].
Aetiology[edit | edit source]
Conditions frequently associated with neuropathic pain can be categorised into two major groups: pain due to damage in the central nervous system (CNS) and pain due to damage to the peripheral nervous system (PNS).
| CNS | PNS |
|---|---|
| Strokes: Cortical and subcortical | Nerve compression/ entrapment neuropathies (carpal tunnel syndrome, thoracic outlet syndrome, nerve root compression |
| Spinal cord injuries | Post-traumatic neuropathy (following surgical procedures or acute injury) |
| Syringomyelia and syringobulbia | Post-amputation stump and phantom limb pain |
| Trigeminal and glossopharyngeal neuralgias | Postherpetic neuralgia |
| Neoplastic and other space-occupying lesions | Disease related neuropathies: Diabetic neuropathy, cancer-related neuropathies and HIV-related neuropathy |
| Central pain in Multiple Sclerosis | Complex regional pain syndrome (CRPS) type 2 |
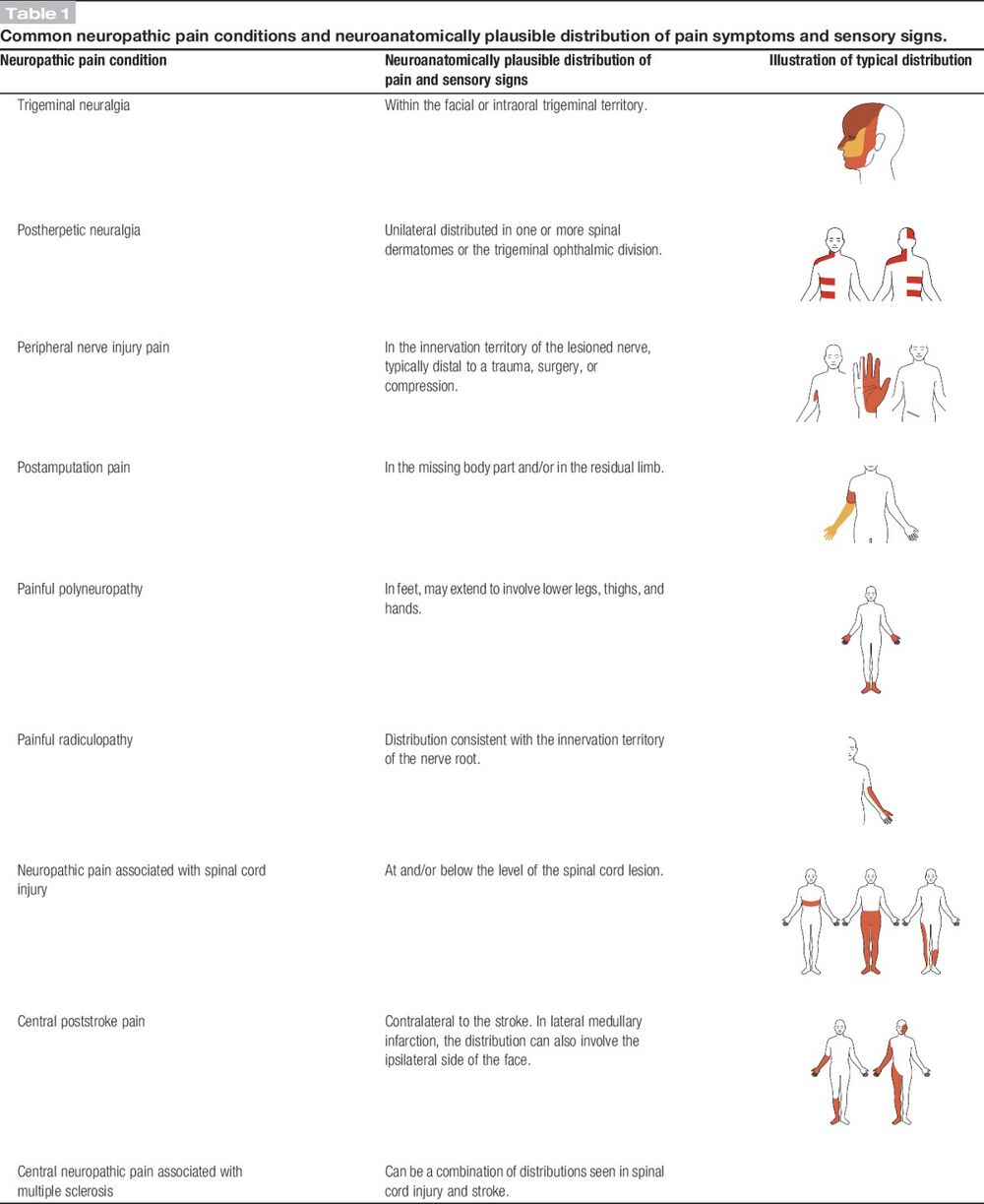 Also see Neuropathies
Also see Neuropathies
Epidemiology[edit | edit source]
The estimated prevalence of neuropathic pain is about 5%, but due to the difficulty in accurately diagnosing neuropathic pain, this number is likely an underestimation[3]. Neuropathic pain is not an inevitable consequence of a neural lesion, and the progression from acute to chronic neuropathic pain occurs only in a minority and is influenced by a huge number of factors[4].
Below is a table presenting the prevalence of neuropathic pain (NP) in various conditions[5]:
| Condition | Prevalence of NP |
|---|---|
| Diabetic Polyneuropathy | 37-42% |
| Brachial Plexus Injury | 56% |
| Postsurgical pain(thoracic/breast surgery) | up to 68% |
| Postsurgical pain (hip/knee arthroplasty) | 6% |
The following risk factors could predispose an individual to the development of chronic neuropathic pain[3]:
- Age 50-64 years
- Being female
- Sociodemographic status: there is some evidence that unemployment and lower educational levels is associated with a higher incidence of neuropathic pain
- Injury sites: some anatomical sites are more prone to developing neuropathic pain
- Emotional and cognitive well-being: can influence how neuropathic pain is experienced
Pathophysiology[edit | edit source]
Neuropathic pain is the result of a disease or lesion of the somatosensory nervous system which results in altered and disordered transmission of sensory signals[3]. Changes in nerve function occur both at the site of the injury and areas around the injury[1]. Peripheral, spinal and central changes result in increased excitability and facilitation of signals, and a loss of inhibition[3]. With increasing chronicity, there is increased involvement of central changes. The pathophysiology is complex and still not fully understood.
| Peripheral changes | Spinal cord changes | Cortical changes | |
|---|---|---|---|
| Spontaneous pain | Ectopic impulses along Aβ, Aδ, and C fibres of neuromas, nerve ends and nerve roots | Ectopic impulses at the dorsal root ganglia | Ectopic impulses at the thalamus |
| Sensitisation | Alterations in ion-channels: increased function of sodium channels leading to increased excitability; loss of potassium channels to modulate nerve activity | Increased expression and function of calcium channels leading to increased transmitter release and enhanced excitability of spinal neurons; expanded receptor fields through NMDA-receptor activation; A-fibre sprouting into laminae I and II of the dorsal horn, which can result in allodynia | Altered descending inhibition at the brain-stem (modulated by the cingulate cortex and amygdala); Limbic areas drive anxiety, depression and sleep problems; cortical reorganisation |
Abnormal Impulse Generating Sites (AIGS)[edit | edit source]
- Abnormal Impulse Generating Sites (AIGS) are defined as the unmyelinated sites along the damaged axon in which the number, kind and excitability of ion channels are altered. This change in ion channel distribution make the site susceptible to non-noxious stimuli such as mechanical, chemical, or thermal stimuli[7][8].
- The high density of ion channels may result in a resting potential close to the threshold, leading to spontaneous ectopic impulses.[9]
- AIGS fire antidromically and orthodromically, resulting in constant noxious stimuli into the central nervous system and neurogenic inflammation in the tissues. For this reason AIGS can persist even though the primary injury on the axon is completely healed.
- Where axons are disconnected from their distal targets (eg. amputation), inflammation and sprouting occur, resulting in neuromas. A neuroma may become an anatomical site for generating nociceptive impulses.
- Together, neuromas, AIGS and dorsal root ganglion changes in receptors can be the peripheral nervous system cause ofphantom limb pain.[10]
Central Sensitisation[edit | edit source]
The term central sensitisation (CS) is often incorrectly used synonymously with neuropathic pain. CS is however a mechanism that can occur independent of nerve lesions, but is often an underlying mechanism involved in chronic neuropathic pain. Central sensitisation is a state where spinal excitability increases due to repeated painful inputs[4]. CS results in decreased thresholds and increased response to nociceptive input.
- Following a peripheral origin, central sensitisation may develop as a result of ectopic neuronal activity in the spinal cord dorsal horn, implying a potential autonomous pain-generating mechanism.[11] This leads to an increase in the size of the sensory receptive field, a reduced threshold for nociception, and hypersensitivity to various innocuous stimuli.
- First order neurons may increase their firing if they are partially damaged and increase the number of sodium channels.
- Ectopic discharges are a result of enhanced depolarization at certain sites in the fibre, leading to spontaneous pain and movement-related pain.
- Inhibitory circuits may be impaired at the level of the dorsal horn or brain stem (or both) allowing pain impulses to travel unopposed. See Pain Facilitation and Inhibition
- In addition, there may be alterations in the central processing of pain when. Second- and third-order neurons develop a “memory” of pain and become sensitised. There is then heightened sensitivity in spinal neurons and reduced activation thresholds. [2]
Clinical Presentation[edit | edit source]
Neuropathic pain is typically characterised by pain that is associated with sensory symptoms/deficits. The coexistence of both positive and negative somatosensory signs is a key diagnostic feature[3]:
| Positive signs | Negative signs | |
|---|---|---|
| Features | Paraesthesia: crawling/tingling sensation
Spontaneous pain: burning, shooting, electric-like sensations Hyperalgesia: increased sensitivity to noxious stimuli Allodynia: pain in response to non-noxious stimuli Summation: progressive worsening of pain with repetitive stimulation |
Hyposthesia: sensory loss and numbness
Sensory loss is in the distribution of the damaged nerve or in the areas that correspond to the spinal/cortical region that has been damaged |
History: Patients will often report spontaneous pain and sensations of ‘pins and needles,’ shooting, burning, stabbing, crawling, and paroxysmal pain (electric-shock like). These sensations affect not only the patient’s sensory system, but also the patient’s well-being, mood and focus. The onset of pain may be delayed, as is often the case in central post-stroke pain, or phantom limb pain where neuropathic symptoms may start months or years after the primary event of injury.
Clinical findings: There may be decreased reflexes, weakness and autonomic changes[4]. Sensation testing will reveal abnormalities - if sensation is normal, it is highly likely that the pain experienced is of neuropathic origin.
Neuropathic pain is often mixed with other pain mechanisms (nociceptive, central sensitisation), which may result in a mixed clinical presentation.
Diagnosis and Assessment[edit | edit source]
One of the challenges of neuropathic pain, is the ability to assess it. Assessing quality, intensity and improvement, as well as accurately diagnosing neuropathic pain is often complex.
There are, however, some diagnostic tools that may assist clinicians in evaluating neuropathic pain. eg nerve conduction studies and sensory-evoked potentials can identify and quantify the extent of damage to sensory (but not nociceptive) pathways by monitoring neurophysiological responses to electrical stimuli.
It is also very important to perform a thorough neurological evaluation to identify motor, sensory and autonomic dysfunctions. Use a paperclip/pin to test pinprick sensation, cotton to test light touch and a cooled reflex hammer to test cold sensation[12].
| Possible NP | Probable NP | Confirmed NP | |
|---|---|---|---|
| History of relevant neurological lesion or disease; pain distribution neuroanatomically plausible | Pain associated with sensory signs in the same neuroanatomically plausible distribution on clinical examination | Diagnostic test confirming a lesion or diseae of the somatosensory nervous system explaining the pain | |
| History and pain and sensory descriptors are compatible with a lesion in the nervous system and not an inflammatory or non-neurological condition | The area of sensory changes may extend beyond, be within, or overlap the area of pain | Requires that other types of pain are excluded or highly unlikely to entirely explain the pain condition |
Quantitative Sensory Testing[edit | edit source]
Quantitative Sensory Testing (QST) examines thermal and mechanical sensory function and can be used to quantify sensory loss or sensory gain. It can be particularly useful when basic sensory testing does not reveal any somatosensory losses, but neuropathic pain is still suspected.
QST is often time consuming and involves expensive equipment, which limits is application in clinical practice. There are however simplified bedside QST tests that can be useful to gain improved understanding related underlying pain mechanisms[3].
Outcome Measures[edit | edit source]
The following tools can be used to screen for neuropathic pain and to monitor neuropathic pain over time[12]:
- DN4 - a well validated screening tool to rank the probability that of peripheral or central neuropathic mechanisms are involved in chronic pain[3]
- LANSS
- PainDETECT
The following outcome measures can be used to quantify the consequences of neuropathic pain: Pain Disability Index, Beck Depression Inventory, HADS, Pain Catastrophising Scale, BPI, EQ-5D, SF-36
Management[edit | edit source]
Neuropathic pain often responds poorly to standard pain treatments. For some people, it can lead to significant disability. Since the cause of neuropathic pain can often not be treated, the focus of its management is on alleviating the symptoms which requires an multidisciplinary approach that combines therapies:
- Medical management and the use of pharmacological agents
- Physiotherapy
- Low impact general physical activities
- Cognitive behavioural therapy[13]
- Relaxation therapy
- Massage therapy
- Acupuncture
Pharmacological Management[edit | edit source]
A number of pharmacological treatments can be used to manage neuropathic pain outside of specialist pain management services. However, there is considerable variation in how treatment is initiated, the dosages used, the order in which drugs are introduced, whether therapeutic doses are achieved and whether there is correct sequencing of therapeutic classes. A further issue is that a number of commonly used treatments are unlicensed for treating neuropathic pain, which may limit their use. These factors may lead to inadequate pain control, with considerable morbidity. The goal of pharmacotherapy is to selectively block the production and/or transmission of pain-producing afferent activity in the periphery, and to engage mechanisms of endogenous inhibition in the CNS[14].
For commonly used pharmacological treatments see Neuropathic Pain Medication [2]. See the summary table below. Note that trigeminal neuralgia presents an exception, where Carbamazepine is regarded as first-line treatment[3].
Recommendations from the Special Interest Group of Neuropathic Pain (NeuPSIG)[15] :
| First line | Second line | Third line |
|---|---|---|
| Gabapentanoids (pregabalin, gabapentin) | Lidocaine patches | Strong opioids |
| Tricyclic antidepressants (TCAs) | Topical capsaicin | Botulinum toxin A |
| Selective serotonin-norepinephrine reuptake inhibitors (SNRIs) | Tramadol |
It is very important to identify concomitant underlying pain mechanisms, as this could improve the effectiveness of pharmacological management by targeting additional mechanisms with different analgesic agents[3].
Other Medical Interventions[edit | edit source]
For some individuals with refractory neuropathic pain, interventional treatments—which deliver medications to specific regions or modify particular brain structures—offer alternative therapy options.
Some of the interventional treatments include:
- Spinal cord stimulation: Requires the application of a monophasic square-wave pulse (at a frequency in the 30–100 Hz range) that results in paraesthesia of the painful part[16][17]
- Cortical stimulation: Using either invasive epidural or transcranial non-invasive procedures (such repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation), cortical stimulation entails stimulating the pre-central motor cortex below the motor threshold.
- Deep brain stimulation: The internal capsule, various nuclei in the sensory thalamus, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus, and anterior cingulate cortex are potential targets for pain relief in deep brain stimulation.
- Intrathecal treatments: In individuals with persistent pain that is severe and otherwise resistant to treatment, this offers a targeted drug delivery option to the painful part.[16]
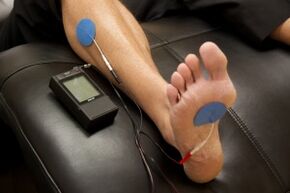
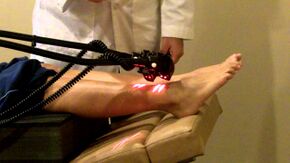
Physiotherapy Management[edit | edit source]
Physiotherapy plays a pivotal role in the complex management of neuropathic pain and must be can considered as an alternative or complementary treatment to pharmacotherapy[13].Physiotherapy aims to address inflammation, stiffness, function and pain with exercise, mobilisation and electrotherapy modalities. Treatment aims to help the body heal itself by encouraging the production of the body's natural pain-relieving chemicals.
- Acute pain relief: for patients who have severe pain, start with contrast baths, desensitisation techniques, and oedema control[14].
- Electrotherapy: TENS therapy has low quality evidence for being effective in the treatment of painful peripheral neuropathy[13][18]. Low level laser therapy may provide pain relief for neuropathic pain [19].
- Therapeutic Exercise: Gentle ROM exercises and strengthening exercises can be useful to reorganise sprouting and cortical body representation, and to restore functional ROM and strength [20][21]. Studies have demonstrated the effectiveness of exercise in improving neuropathic pain in spinal cord injuries, stroke, multiple sclerosis, radiculopathy, diabetic polyneuropathy and HIV-related neuropathy [22].
- Graded motor imagery: Mirror therapy and graded motor imagery are movement representation techniques and have been suggested to be beneficial in neuropathic pain management - especially for phantom limb pain , CRPS and stroke pain[23].
- Aerobic Exercise: Regular aerobic exercise has been shown to help manage chronic pain and can even help with nerve recovery[22]. There are studies showing that exercise may be an important part of the treatment and prevention of neuropathic pain after chemotherapy[24]. Aerobic exercise resulted in improved mechanical allodynia and heat hyperalgesia in animal models of neuropathic pain [25]. The positive effects of exercise can be, at least in part, be explained by the stimulation of the endogenous opioid system resulting in exercise-induced hypoaglesia[22].
Nutrition is also an important factor when managing neuropathic pain. In this video, Dr. Andrea Furlan outlines 7 food groups to include if you have neuropathic pain, and 5 food groups to avoid.
Multi-disciplinary Approach[edit | edit source]
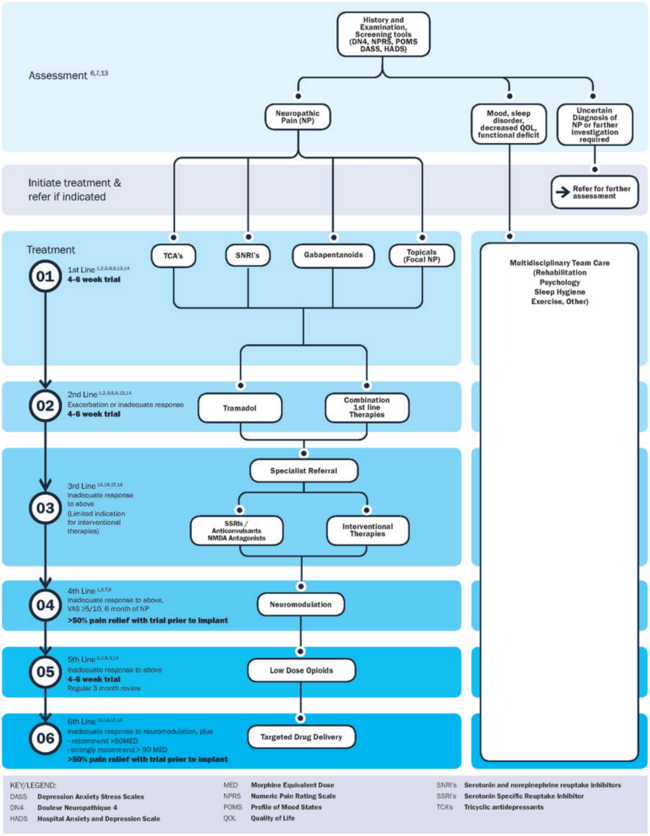
All rehabilitation professionals and clinicians can play in important role in identifying and diagnosing neuropathic pain. The complex nature of neuropathic pain calls for an integrated team approach. Rehabilitation can often not occur effectively without adequate pharmacological pain management, emphasising the importance of a team approach. Psychosocial aspects should not be undermined, with a focus on improving participation and quality of life[13] . Multi-disciplinary care of neuropathic pain has been shown to significantly decrease pain, improve function, mood and pain acceptance[12]. A patient-specific balance of pharmacological, psychological and physical management strategies need to be integrated. See the Physiopedia pages for each specific condition for more detailed integrated management approaches.
A proposed comprehensive treatment algorithm for the management of neuropathic pain by Bates et al, 2019[12]:
Please watch the following video for relevant infomration about neuropathic pain.
Management of Neuropathic Pain[27]
Resources[edit | edit source]
Exercise Recommendations for specific neuropathic conditions
References[edit | edit source]
- ↑ 1.0 1.1 PPM The Pathophysiology of Neuropathic Pain Available:https://www.practicalpainmanagement.com/pain/neuropathic/pathophysiology-neuropathic-pain (accessed 30.10.2021)
- ↑ 2.0 2.1 2.2 NICE. Neuropathic pain in adults: pharmacological management in non-specialist settings.Available: https://www.ncbi.nlm.nih.gov/books/NBK552848/(accessed 30.9.2021)
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annual Review of Pharmacology and Toxicology. 2020 Jan 6;60:257-74.
- ↑ 4.0 4.1 4.2 4.3 4.4 Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiological reviews. 2020 Nov 3.
- ↑ Di Stefano G, Di Lionardo A, Di Pietro G, Truini A. Neuropathic pain related to peripheral neuropathies according to the iasp grading system criteria. Brain Sciences. 2020 Dec 22;11(1):1.
- ↑ Ochoa J, Torebjork HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–854.
- ↑ Greening J, Lynn B. Minor peripheral nerve injuries an underestimated source of pain? Manual Therapy, 1998; 3: 187-194
- ↑ Gifford L. Acute low cervical nerve root conditions: symptom presentation and pathobiological reasoning. Manual Therapy, 2001; 6: 106-115
- ↑ Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion — a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38
- ↑ Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168-2176.
- ↑ Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS . Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain, 2014; 155 (7): 1272-9
- ↑ 12.0 12.1 12.2 12.3 Bates D, Schultheis BC, Hanes MC, Jolly SM, Chakravarthy KV, Deer TR, Levy RM, Hunter CW. A comprehensive algorithm for management of neuropathic pain. Pain Medicine. 2019 Jun 1;20(Supplement_1):S2-12.
- ↑ 13.0 13.1 13.2 13.3 Bernetti A, Agostini F, de Sire A, Mangone M, Tognolo L, Di Cesare A, Ruiu P, Paolucci T, Invernizzi M, Paoloni M. Neuropathic pain and rehabilitation: a systematic review of international guidelines. Diagnostics. 2021 Jan 5;11(1):74.
- ↑ 14.0 14.1 Zusman M. Mechanisms of peripheral neuropathic pain: implications for musculoskeletal physiotherapy. Physical Therapy Reviews. 2008 Oct 1;13(5):313-23.
- ↑ Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. International Journal of Immunopathology and Pharmacology. 2019 Mar;33:2058738419838383.
- ↑ 16.0 16.1 Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C. Neuropathic pain. Nature reviews Disease primers. 2017 Feb 16;3(1):1-9.
- ↑ Dones I, Levi V. Spinal cord stimulation for neuropathic pain: current trends and future applications. Brain sciences. 2018 Jul 24;8(8):138.
- ↑ Gibson W, Wand BM, O'Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. 2017 Sep 14;9(9):CD011976
- ↑ de Andrade AL, Bossini PS, Parizotto NA. Use of low level laser therapy to control neuropathic pain: A systematic review. J Photochem Photobiol B. 2016 Nov;164:36-42.
- ↑ Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001 Dec;65(12):1378-82.
- ↑ Trout KK. The neuromatrix theory of pain: implications for selected nonpharmacologic methods of pain relief for labor. J Midwifery Womens Health. 2004 Nov-Dec;49(6):482-8.
- ↑ 22.0 22.1 22.2 Zhang YH, Hu HY, Xiong YC, Peng C, Hu L, Kong YZ, Wang YL, Guo JB, Bi S, Li TS, Ao LJ. Exercise for neuropathic pain: a systematic review and expert consensus. Frontiers in Medicine. 2021 Nov 24;8:756940.
- ↑ Kim SY, Kim YY. Mirror therapy for phantom limb pain. Korean J Pain. 2012;25(4):272-274.
- ↑ Majithia N, Loprinzi CL, Smith TJ.New Practical Approaches to Chemotherapy-Induced Neuropathic Pain: Prevention, Assessment, and Treatment. Oncology (Williston Park). 2016 Nov 15;30(11)
- ↑ Kami K, Tajima F, Senba E. Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain. Anat Sci Int. 2016 Aug 2. [Epub ahead of print]
- ↑ Dr. Andrea Furlan. #131 Seven Foods to improve NERVE PAIN and five foods and 5 to avoid if you have NEUROPATHIC pain. Available from: https://www.youtube.com/watch?v=clbpGLcg_dM&ab_channel=Dr.AndreaFurlan (accessed 17 September 2023).
- ↑ Management of Neuropathic Pain. Available from: https://www.youtube.com/watch?v=hDu_WdRNDzo&t=796s