Neuropathic Pain: Difference between revisions
(Added content) |
No edit summary |
||
| Line 19: | Line 19: | ||
== Aetiology == | == Aetiology == | ||
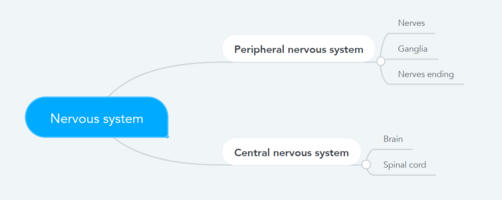
Conditions frequently associated with neuropathic pain can be categorised into two major groups: pain due to damage in the central nervous system (CNS) and pain due to damage to the peripheral nervous system (PNS). | Conditions frequently associated with neuropathic pain can be categorised into two major groups: pain due to damage in the central nervous system (CNS) and pain due to damage to the peripheral nervous system (PNS). | ||
[[File:Anatomical division of the nervous system.png|502x502px|alt=|thumb|Anatomical division of the nervous system|left]] | |||
{| class="wikitable" | {| class="wikitable" | ||
|+'''Conditions associated with neuropathic pain''' | |+'''Conditions associated with neuropathic pain''' | ||
| Line 42: | Line 42: | ||
|- | |- | ||
| | | | ||
|Complex regional pain syndrome (CRPS) | |Complex regional pain syndrome (CRPS) type 2 | ||
|} | |} | ||
Also see '''[[Neuropathies]]''' | Also see '''[[Neuropathies]]''' | ||
| Line 56: | Line 56: | ||
* Injury sites: some anatomical sites are more prone to developing neuropathic pain | * Injury sites: some anatomical sites are more prone to developing neuropathic pain | ||
* Emotional and cognitive well-being: can influence how neuropathic pain is experienced | * Emotional and cognitive well-being: can influence how neuropathic pain is experienced | ||
== Pathophysiology == | == Pathophysiology == | ||
Neuropathic pain is the result of disease or | Neuropathic pain is the result of disease or lesion of the somatosensory nervous system which results in altered and disordered transmission of sensory signals<ref name=":4" />. Changes in nerve function occur both at the site of the injury and areas around the injury<ref name=":1" />. Peripheral, spinal and central changes result in increases excitability and facilitation of signals, and a loss of inhibition<ref name=":4" />. With increasing chronicity, there is increased involvement of central changes. | ||
{| class="wikitable" | |||
|+Pathophysiology in Neuropathic pain<ref name=":4" /><ref>Ochoa J, Torebjork HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–854.</ref><ref>Finnerup NB, Kuner R, Jensen TS. [[Neuropathic pain: from mechanisms to treatment.]] Physiological reviews. 2020 Nov 3.</ref> | |||
! | |||
!'''Peripheral changes''' | |||
!'''Spinal cord changes''' | |||
!'''Cortical changes''' | |||
|- | |||
|'''Spontaneous pain''' | |||
|Ectopic impulses along Aβ, Aδ, and C fibres of neuromas, nerves and nerve roots | |||
|Ectopic impulses at the dorsal root ganglia | |||
|Ectopic impulses at the thalamus | |||
|- | |||
|'''Sensitisation''' | |||
|Alterations in ion-channels: increased function of sodium channels leading to increased excitability, loss of potassium channels to modulate nerve activity | |||
|Increased expression and function of calcium channels leading to increased transmitter release and enhanced excitability of spinal neurons | |||
|Altered descending inhibition at the brain-stem | |||
|- | |||
| | |||
| | |||
| | |||
| | |||
|- | |||
| | |||
| | |||
|A-fibre sprouting into laminae I and II of the dorsal horn, which can result in allodynia | |||
|Limbic areas drive anxiety, depression and sleep problems | |||
|} | |||
=== Central Sensitisation === | |||
The term central sensitisation (CS) is often used synonymy with neuropathic pain. CS is however a mechanism that can occur independent of nerve lesions. The mechanisms involved in neuropathic pain, can however often give rise to central sensitisation. | |||
* Following a peripheral origin, central sensitisation may develop as a result of ectopic neuronal activity in the [[Spinal cord anatomy|spinal cord]] dorsal horn, implying a potential autonomous pain-generating mechanism.<ref name=":2">Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS . [https://pubmed.ncbi.nlm.nih.gov/24704366/ Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy.] Pain, 2014; 155 (7): 1272-9</ref> Thus may contribute to an increase in the size of the sensory receptive field, to reduced threshold for pain perception, and hypersensitivity to various innocuous stimuli in the dorsal horn. | |||
* Following a peripheral origin, central | |||
* The antidromic direction of the impulse generated in the AIGS can maintain the inflammation of the tissues. This may subdue the chronic chemical stimuli of the peripheral nociceptive pain. | * The antidromic direction of the impulse generated in the AIGS can maintain the inflammation of the tissues. This may subdue the chronic chemical stimuli of the peripheral nociceptive pain. | ||
* First order [[Neurone|neuron]]<nowiki/>s may increase their firing if they are partially damaged and increase the number of sodium channels. | * First order [[Neurone|neuron]]<nowiki/>s may increase their firing if they are partially damaged and increase the number of sodium channels. | ||
| Line 82: | Line 96: | ||
* Inhibitory [[Interneurons|circuits]] may be impaired at the level of the dorsal horn or [[Brainstem|brain stem]] (or both) allowing pain impulses to travel unopposed. See [[Pain Facilitation and Inhibition]] | * Inhibitory [[Interneurons|circuits]] may be impaired at the level of the dorsal horn or [[Brainstem|brain stem]] (or both) allowing pain impulses to travel unopposed. See [[Pain Facilitation and Inhibition]] | ||
* In addition, there may be alterations in the central processing of pain when, (due to [[Chronic Pain and the Brain|chronic pain]] and the use of some drugs), second- and third-order neurons develop a “memory” of pain and become sensitized. There is then heightened sensitivity in spinal neurons and reduced activation thresholds. <ref name=":0" /> | * In addition, there may be alterations in the central processing of pain when, (due to [[Chronic Pain and the Brain|chronic pain]] and the use of some drugs), second- and third-order neurons develop a “memory” of pain and become sensitized. There is then heightened sensitivity in spinal neurons and reduced activation thresholds. <ref name=":0" /> | ||
he main substrate for the central sensitization is activation of the ''N-''methyl-d-aspartate (NMDA) receptor for glutamate (21). Central sensitization is a state where spinal excitability increases due to repeated painful inputs. In neuropathic conditions, hyperexcitable spinal neurons exhibit increased responses to many sensory modalities and expand their receptive fields. This is the most plausible explanation for dynamic, static, and cold allodynias and is reflected by enhanced thalamic neuronal coding (22). Hyperexcitability is compounded by a loss of GABA-mediated inhibitions at spinal levels (23), and there are less well understood functional changes in nonneuronal signaling within the spinal cord (24). | |||
Consequently, the brain receives altered and abnormal sensory messages. Areas such as the cingulate cortex and amygdala have been implicated in the ongoing aversive state and comorbidities associated with NeP (25). Projections from these forebrain areas now alter descending controls running from the periaqueductal gray to the brain stem. Under normal conditions, there is a balance between descending inhibition and excitation, but after peripheral neuropathy, the latter dominates. Several studies have shown that the brainstem excitatory pathways are more important in the maintenance rather than induction of the pain state (26–29). | |||
Increased NMDA receptor activity contributes to the process of central sensitization (40) in certain types of NeP (41, 42) owing to its crucial role in nociceptive transmission and synaptic plasticity, reflected in the human wind-up ratio. | |||
In total, activity-dependent plasticity in the periphery and brain/spinal cord cumulatively represents the major pathophysiological mechanism contributing to NeP. Various synaptic modulators, excitatory amino acids, and alterations in ion channel kinetics synergize to increase synaptic strength. Responses of nociceptive neurons are enhanced and driven by subthreshold inputs, and receptive fields are enlarged. Central sensitization is triggered by ongoing activity originating in injured nerves and amplification within the CNS | |||
n the clinic, NeP is not an inevitable consequence of neural lesion, and the progression from acute to chronic neuropathy occurs only in a minority and is influenced by a huge number of factors as varied as the age of the nervous system (65) and, for example, operative approach (for persistent postsurgical pains) (66). | |||
See also [https://www.physio-pedia.com/images/d/db/NeuropathicPain.hysio.pdf Mechanisms of peripheral neuropathic pain: implications for musculoskeletal physiotherapy] | See also [https://www.physio-pedia.com/images/d/db/NeuropathicPain.hysio.pdf Mechanisms of peripheral neuropathic pain: implications for musculoskeletal physiotherapy] | ||
=== Abnormal Impulse Generating Sites (AIGS) === | |||
* Following a peripheral origin, [[Central Sensitisation|central sensitisation]] may be sustained as a result of ectopic neuronal activity both in peripheral nerves and, as a consequence, in dorsal root ganglion and in the dorsal horn of the spinal cord. | |||
* Abnormal Impulse Generating Sites (AIGS) are defined as the unmyelinated sites along the damaged axon in which the number, kind and excitability of ion channels are altered. This change in ion channel distribution make the site susceptible to non-noxious stimuli such as mechanical, chemical, or thermal stimuli<ref>Greening J, Lynn B. Minor peripheral nerve injuries an underestimated source of pain? Manual Therapy, 1998; 3: 187-194</ref><ref>Gifford L. Acute low cervical nerve root conditions: symptom presentation and pathobiological reasoning. Manual Therapy, 2001; 6: 106-115</ref>. | |||
* Ectopic impulses may also been generated spontaneously. In fact the high density of ion channels may result in a resting potential close to the threshold.<ref>Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion — a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38</ref> | |||
* When an injury occurs to the nerves, neurons in the dorsal root ganglion increase their nociceptive signalling through increases in neuronal excitability and the creation of ectopic discharges. This is consequent of profound changes in the distribution of the receptors on dorsal root ganglion cell bodies. | |||
* Following limb amputation for examples, the axons are disconnected from their distal targets and inflammation and sprouting occur in the resulting residual limb, where a neuroma can form. A neuroma may indeed represent an anatomical site for generating nociceptive impulses. | |||
* Together, neuromas, AIGS and dorsal root ganglion change in receptors can be the peripheral nervous system cause of the [[Phantom Limb Pain|phantom limb pain.]]<ref>Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. ''J Clin Invest''. 2018;128(6):2168-2176.</ref> | |||
* AIGS fires antidromically and orthodromically, resulting in constant noxious stimulus into the central nervous system and [[Neurogenic Inflammation|neurogenic inflammation]] in the tissues. For this reason AIGS can persist even though the primary injury on the axon is completely healed. | |||
== Clinical Presentation == | == Clinical Presentation == | ||
| Line 89: | Line 123: | ||
{| class="wikitable" | {| class="wikitable" | ||
! | ! | ||
!'''Positive signs''' | |||
!'''Negative signs''' | !'''Negative signs''' | ||
|- | |- | ||
|''Explanation'' | |''Explanation'' | ||
|AIGs | |||
|Sensory deficits to noxious and thermal stimuli, caused by damage to small-diameter afferent fibres | |Sensory deficits to noxious and thermal stimuli, caused by damage to small-diameter afferent fibres | ||
|- | |- | ||
|''Features'' | |''Features'' | ||
|'''Paraesthesia:''' crawling/tingling sensation | |'''Paraesthesia:''' crawling/tingling sensation | ||
'''Spontaneous pain:''' burning, shooting, electric-like sensations | '''Spontaneous pain:''' burning, shooting, electric-like sensations | ||
| Line 106: | Line 139: | ||
'''Summation:''' progressive worsening of pain with repetitive stimulation | '''Summation:''' progressive worsening of pain with repetitive stimulation | ||
|Hyposthesia: sensory loss and numbness | |||
Sensory loss is in the distribution of the damaged nerve or in the areas that correspond to the spinal/cortical region that has been damaged | |||
|} | |} | ||
| Line 123: | Line 158: | ||
It is also very important to perform a thorough [[Neurological Assessment|neurological]] evaluation to identify motor, sensory and autonomic dysfunctions. Finally, there are numerous questionnaires used to distinguish neuropathic pain from nociceptive pain<ref name=":1" />.e.g., the Neuropathic Questionnaire11 and ID Pain. | It is also very important to perform a thorough [[Neurological Assessment|neurological]] evaluation to identify motor, sensory and autonomic dysfunctions. Finally, there are numerous questionnaires used to distinguish neuropathic pain from nociceptive pain<ref name=":1" />.e.g., the Neuropathic Questionnaire11 and ID Pain. | ||
=== Quantitative Sensory Testing === | |||
QST is often time consuming and involved expensive equipment, which limits is application in clinical practice. There are however simplified bedside QST tests that can be useful to gain improved understanding related underlying pain mechanisms<ref name=":4" />. | |||
=== Outcome Measures === | === Outcome Measures === | ||
| Line 132: | Line 170: | ||
== Management == | == Management == | ||
Neuropathic pain often responds poorly to standard pain treatments and occasionally may worse instead of better over time. For some people, it can lead to | Neuropathic pain often responds poorly to standard pain treatments and occasionally may get worse instead of better over time. For some people, it can lead to significant disability. Since the cause of neuropathic pain can often not be treated, the focus of its management is on alleviating the symptoms which requires an multidisciplinary approach that combines therapies: [[File:Neuro-HTtens-300x200.jpg|alt=|TENS therapy|thumb]] | ||
# Medical management and the use of pharmacological agents | # Medical management and the use of pharmacological agents | ||
# | # Physiotherapy | ||
# Low impact general physical activities | # Low impact general physical activities | ||
# Counselling | # Counselling | ||
# Relaxation therapy | # Relaxation therapy | ||
# [[Massage|Massage therapy]] | # [[Massage|Massage therapy]] | ||
# [[Acupuncture]] | # [[Acupuncture]] | ||
== | === Pharmacological Management === | ||
A number of pharmacological treatments can be used to manage neuropathic pain outside of specialist pain management services. However, there is considerable variation in how treatment is initiated, the dosages used and the order in which drugs are introduced, whether therapeutic doses are achieved and whether there is correct sequencing of therapeutic classes. A further issue is that a number of commonly used treatments are unlicensed for treating neuropathic pain, which may limit their use. These factors may lead to inadequate pain control, with considerable morbidity. | A number of pharmacological treatments can be used to manage neuropathic pain outside of specialist pain management services. However, there is considerable variation in how treatment is initiated, the dosages used and the order in which drugs are introduced, whether therapeutic doses are achieved and whether there is correct sequencing of therapeutic classes. A further issue is that a number of commonly used treatments are unlicensed for treating neuropathic pain, which may limit their use. These factors may lead to inadequate pain control, with considerable morbidity. | ||
For commonly used pharmacological treatments see [[Neuropathic Pain Medication]] <ref name=":0" />. | For commonly used pharmacological treatments see [[Neuropathic Pain Medication]] <ref name=":0" />. '''First line''' treatment generally includes: Tricyclic anti-depressants (TCAs), Serotonin-reuptake inhibitors (SNRIs), Pregabalin, Gabapentin<ref name=":4" />. Carbamazepine is however regarded as the first-line treatment for trigeminal neuralgia. | ||
It is very important to identify concomitant underlying pain mechanisms, as this could improve the effectiveness of pharmacological management by targeting additional mechanisms with different analgesic agents<ref name=":4" />. | |||
=== Other Medical Interventions === | |||
For some individuals with refractory neuropathic pain, interventional treatments—which deliver medications to specific regions or modify particular brain structures—offer alternative therapy options. | For some individuals with refractory neuropathic pain, interventional treatments—which deliver medications to specific regions or modify particular brain structures—offer alternative therapy options. | ||
Some of the interventional treatments | Some of the interventional treatments include: | ||
# Cortical stimulation: Using either invasive epidural or transcranial non-invasive procedures (such repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation), cortical stimulation entails stimulating the pre-central motor cortex below the motor threshold. | # '''Spinal cord stimulation:''' Requires the application of a monophasic square-wave pulse (at a frequency in the 30–100 Hz range) that results in paraesthesia of the painful part<ref name=":3">Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C. [https://www.nature.com/articles/nrdp20172 Neuropathic pain]. Nature reviews Disease primers. 2017 Feb 16;3(1):1-9.</ref><ref>Dones I, Levi V. S[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6119923/#:~:text=Among%20them%2C%20spinal%20cord%20stimulation,II%2C%20postherpetic%20neuralgia%20and%20pure pinal cord stimulation for neuropathic pain: current trends and future applications.] Brain sciences. 2018 Jul 24;8(8):138.</ref> | ||
# Deep brain stimulation: The internal capsule, various nuclei in the sensory thalamus, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus, and anterior cingulate cortex are potential targets for pain relief in deep brain stimulation. | # '''Cortical stimulation:''' Using either invasive epidural or transcranial non-invasive procedures (such repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation), cortical stimulation entails stimulating the pre-central motor cortex below the motor threshold. | ||
# Intrathecal treatments : In individuals with persistent pain that is severe and otherwise resistant to treatment, this offers a targeted drug delivery option to the painful part.<ref name=":3" /> | # '''Deep brain stimulation:''' The internal capsule, various nuclei in the sensory thalamus, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus, and anterior cingulate cortex are potential targets for pain relief in deep brain stimulation. | ||
# '''Intrathecal treatments:''' In individuals with persistent pain that is severe and otherwise resistant to treatment, this offers a targeted drug delivery option to the painful part.<ref name=":3" /> | |||
=== Physical Therapy Management === | === Physical Therapy Management === | ||
Revision as of 15:01, 18 August 2023
Original Editor - Kerstin McPherson
Top Contributors - Andeela Hafeez, Melissa Coetsee, Kerstin McPherson, Vanessa Rhule, Riccardo Ugrin, Lucinda hampton, Kim Jackson, Admin, Evan Thomas, Chrysolite Jyothi Kommu, Kapil Narale, Temitope Olowoyeye, Jo Etherton, WikiSysop, Vidya Acharya, Lauren Lopez, Amanda Ager, 127.0.0.1, Wendy Walker, Carina Therese Magtibay and Oyemi Sillo
Introduction[edit | edit source]
The International Association for the Study of Pain (2011) defines neuropathic pain as ‘pain caused by a lesion or disease of the somatosensory nervous system’. It is a complex, chronic pain state that presents a challenge to patients and clinicians.
It is the result of disease or damage anywhere along the neuraxis of the peripheral or central (spinal and supraspinal) nervous system[1].
- Central neuropathic pain is defined as ‘pain caused by a lesion or disease of the central somatosensory nervous system’.
- Peripheral neuropathic pain is defined as ‘pain caused by a lesion or disease of the peripheral somatosensory nervous system’.
Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies, symptoms, and underlying mechanisms[2].
Aetiology[edit | edit source]
Conditions frequently associated with neuropathic pain can be categorised into two major groups: pain due to damage in the central nervous system (CNS) and pain due to damage to the peripheral nervous system (PNS).
| CNS | PNS |
|---|---|
| Strokes: Cortical and subcortical | Nerve compression/ entrapment neuropathies (carpal tunnel syndrome, thoracic outlet syndrome, nerve root compression |
| Spinal cord injuries | Post-traumatic neuropathy (following surgical procedures or acute injury) |
| Syringomyelia and syringobulbia | Post-amputation stump and phantom limb pain |
| Trigeminal and glossopharyngeal neuralgias | Postherpetic neuralgia |
| Neoplastic and other space-occupying lesions | Disease related neuropathies: Diabetic neuropathy, cancer-related neuropathies and HIV-related neuropathy |
| Complex regional pain syndrome (CRPS) type 2 |
Also see Neuropathies
Epidemiology[edit | edit source]
The estimated prevalence of neuropathic pain is about 5%, but due to the difficulty in accurately diagnosing neuropathic pain, this number is likely an underestimation[3].
The following risk factors could predispose an individual to the development of neuropathic pain[3]:
- Age 50-64 years
- Being female
- Sociodemographic status: there is some evidence that unemployment and lower educational levels is associated with a higher incidence of neuropathic pain
- Injury sites: some anatomical sites are more prone to developing neuropathic pain
- Emotional and cognitive well-being: can influence how neuropathic pain is experienced
Pathophysiology[edit | edit source]
Neuropathic pain is the result of disease or lesion of the somatosensory nervous system which results in altered and disordered transmission of sensory signals[3]. Changes in nerve function occur both at the site of the injury and areas around the injury[1]. Peripheral, spinal and central changes result in increases excitability and facilitation of signals, and a loss of inhibition[3]. With increasing chronicity, there is increased involvement of central changes.
| Peripheral changes | Spinal cord changes | Cortical changes | |
|---|---|---|---|
| Spontaneous pain | Ectopic impulses along Aβ, Aδ, and C fibres of neuromas, nerves and nerve roots | Ectopic impulses at the dorsal root ganglia | Ectopic impulses at the thalamus |
| Sensitisation | Alterations in ion-channels: increased function of sodium channels leading to increased excitability, loss of potassium channels to modulate nerve activity | Increased expression and function of calcium channels leading to increased transmitter release and enhanced excitability of spinal neurons | Altered descending inhibition at the brain-stem |
| A-fibre sprouting into laminae I and II of the dorsal horn, which can result in allodynia | Limbic areas drive anxiety, depression and sleep problems |
Central Sensitisation[edit | edit source]
The term central sensitisation (CS) is often used synonymy with neuropathic pain. CS is however a mechanism that can occur independent of nerve lesions. The mechanisms involved in neuropathic pain, can however often give rise to central sensitisation.
- Following a peripheral origin, central sensitisation may develop as a result of ectopic neuronal activity in the spinal cord dorsal horn, implying a potential autonomous pain-generating mechanism.[6] Thus may contribute to an increase in the size of the sensory receptive field, to reduced threshold for pain perception, and hypersensitivity to various innocuous stimuli in the dorsal horn.
- The antidromic direction of the impulse generated in the AIGS can maintain the inflammation of the tissues. This may subdue the chronic chemical stimuli of the peripheral nociceptive pain.
- First order neurons may increase their firing if they are partially damaged and increase the number of sodium channels.
- Ectopic discharges are a result of enhanced depolarization at certain sites in the fiber, leading to spontaneous pain and movement-related pain.
- Inhibitory circuits may be impaired at the level of the dorsal horn or brain stem (or both) allowing pain impulses to travel unopposed. See Pain Facilitation and Inhibition
- In addition, there may be alterations in the central processing of pain when, (due to chronic pain and the use of some drugs), second- and third-order neurons develop a “memory” of pain and become sensitized. There is then heightened sensitivity in spinal neurons and reduced activation thresholds. [2]
he main substrate for the central sensitization is activation of the N-methyl-d-aspartate (NMDA) receptor for glutamate (21). Central sensitization is a state where spinal excitability increases due to repeated painful inputs. In neuropathic conditions, hyperexcitable spinal neurons exhibit increased responses to many sensory modalities and expand their receptive fields. This is the most plausible explanation for dynamic, static, and cold allodynias and is reflected by enhanced thalamic neuronal coding (22). Hyperexcitability is compounded by a loss of GABA-mediated inhibitions at spinal levels (23), and there are less well understood functional changes in nonneuronal signaling within the spinal cord (24).
Consequently, the brain receives altered and abnormal sensory messages. Areas such as the cingulate cortex and amygdala have been implicated in the ongoing aversive state and comorbidities associated with NeP (25). Projections from these forebrain areas now alter descending controls running from the periaqueductal gray to the brain stem. Under normal conditions, there is a balance between descending inhibition and excitation, but after peripheral neuropathy, the latter dominates. Several studies have shown that the brainstem excitatory pathways are more important in the maintenance rather than induction of the pain state (26–29).
Increased NMDA receptor activity contributes to the process of central sensitization (40) in certain types of NeP (41, 42) owing to its crucial role in nociceptive transmission and synaptic plasticity, reflected in the human wind-up ratio.
In total, activity-dependent plasticity in the periphery and brain/spinal cord cumulatively represents the major pathophysiological mechanism contributing to NeP. Various synaptic modulators, excitatory amino acids, and alterations in ion channel kinetics synergize to increase synaptic strength. Responses of nociceptive neurons are enhanced and driven by subthreshold inputs, and receptive fields are enlarged. Central sensitization is triggered by ongoing activity originating in injured nerves and amplification within the CNS
n the clinic, NeP is not an inevitable consequence of neural lesion, and the progression from acute to chronic neuropathy occurs only in a minority and is influenced by a huge number of factors as varied as the age of the nervous system (65) and, for example, operative approach (for persistent postsurgical pains) (66).
See also Mechanisms of peripheral neuropathic pain: implications for musculoskeletal physiotherapy
Abnormal Impulse Generating Sites (AIGS)[edit | edit source]
- Following a peripheral origin, central sensitisation may be sustained as a result of ectopic neuronal activity both in peripheral nerves and, as a consequence, in dorsal root ganglion and in the dorsal horn of the spinal cord.
- Abnormal Impulse Generating Sites (AIGS) are defined as the unmyelinated sites along the damaged axon in which the number, kind and excitability of ion channels are altered. This change in ion channel distribution make the site susceptible to non-noxious stimuli such as mechanical, chemical, or thermal stimuli[7][8].
- Ectopic impulses may also been generated spontaneously. In fact the high density of ion channels may result in a resting potential close to the threshold.[9]
- When an injury occurs to the nerves, neurons in the dorsal root ganglion increase their nociceptive signalling through increases in neuronal excitability and the creation of ectopic discharges. This is consequent of profound changes in the distribution of the receptors on dorsal root ganglion cell bodies.
- Following limb amputation for examples, the axons are disconnected from their distal targets and inflammation and sprouting occur in the resulting residual limb, where a neuroma can form. A neuroma may indeed represent an anatomical site for generating nociceptive impulses.
- Together, neuromas, AIGS and dorsal root ganglion change in receptors can be the peripheral nervous system cause of the phantom limb pain.[10]
- AIGS fires antidromically and orthodromically, resulting in constant noxious stimulus into the central nervous system and neurogenic inflammation in the tissues. For this reason AIGS can persist even though the primary injury on the axon is completely healed.
Clinical Presentation[edit | edit source]
Neuropathic pain is typically characterised by pain that is associated with sensory symptoms/deficits. The coexistence of both positive and negative somatosensory signs is a key diagnostic feature[3]:
| Positive signs | Negative signs | |
|---|---|---|
| Explanation | AIGs | Sensory deficits to noxious and thermal stimuli, caused by damage to small-diameter afferent fibres |
| Features | Paraesthesia: crawling/tingling sensation
Spontaneous pain: burning, shooting, electric-like sensations Hyperalgesia: increased sensitivity to noxious stimuli Allodynia: pain in response to non-noxious stimuli (eg. light stroking) Summation: progressive worsening of pain with repetitive stimulation |
Hyposthesia: sensory loss and numbness
Sensory loss is in the distribution of the damaged nerve or in the areas that correspond to the spinal/cortical region that has been damaged |
Patients will often report spontaneous pain and sensations of ‘pins and needles,’ shooting, burning, stabbing, crawling, and paroxysmal pain (electric-shock like). These sensations affect not only the patient’s sensory system, but also the patient’s well-being, mood and focus.
The onset of pain may be delayed, as is often the case in central post-stroke pain, or the phantom limb pain where neuropathic symptoms may start months or years after the primary event of injury.
Neuropathic pain is often mixed with other pain mechanisms (nociceptive, central sensitisation), which may result in a mixed clinical presentation.
Assessment[edit | edit source]
One of the challenges, in regards to neuropathic pain, is the ability to assess it. There is a dual component to this: assessing quality, intensity and improvement; and accurately diagnosing neuropathic pain.
There are, however, some diagnostic tools that may assist clinicians in evaluating neuropathic pain. eg nerve conduction studies and sensory-evoked potentials can identify and quantify the extent of damage to sensory, but not nociceptive, pathways by monitoring neurophysiological responses to electrical stimuli.
Mechanical sensitivity to tactile stimuli is measured with von Frey hairs, pinprick with weighted needles, vibration sensitivity with vibrometers and thermal pain with thermodes.
It is also very important to perform a thorough neurological evaluation to identify motor, sensory and autonomic dysfunctions. Finally, there are numerous questionnaires used to distinguish neuropathic pain from nociceptive pain[1].e.g., the Neuropathic Questionnaire11 and ID Pain.
Quantitative Sensory Testing[edit | edit source]
QST is often time consuming and involved expensive equipment, which limits is application in clinical practice. There are however simplified bedside QST tests that can be useful to gain improved understanding related underlying pain mechanisms[3].
Outcome Measures[edit | edit source]
The following tools can be used to screen for neuropathic pain and to monitor neuropathic pain over time
- DN4 - a well validated screening tool to rank the probability that of peripheral or central neuropathic mechanisms are involved in chronic pain[3]
- LANSS
- PainDETECT
Management[edit | edit source]
Neuropathic pain often responds poorly to standard pain treatments and occasionally may get worse instead of better over time. For some people, it can lead to significant disability. Since the cause of neuropathic pain can often not be treated, the focus of its management is on alleviating the symptoms which requires an multidisciplinary approach that combines therapies:
- Medical management and the use of pharmacological agents
- Physiotherapy
- Low impact general physical activities
- Counselling
- Relaxation therapy
- Massage therapy
- Acupuncture
Pharmacological Management[edit | edit source]
A number of pharmacological treatments can be used to manage neuropathic pain outside of specialist pain management services. However, there is considerable variation in how treatment is initiated, the dosages used and the order in which drugs are introduced, whether therapeutic doses are achieved and whether there is correct sequencing of therapeutic classes. A further issue is that a number of commonly used treatments are unlicensed for treating neuropathic pain, which may limit their use. These factors may lead to inadequate pain control, with considerable morbidity.
For commonly used pharmacological treatments see Neuropathic Pain Medication [2]. First line treatment generally includes: Tricyclic anti-depressants (TCAs), Serotonin-reuptake inhibitors (SNRIs), Pregabalin, Gabapentin[3]. Carbamazepine is however regarded as the first-line treatment for trigeminal neuralgia.
It is very important to identify concomitant underlying pain mechanisms, as this could improve the effectiveness of pharmacological management by targeting additional mechanisms with different analgesic agents[3].
Other Medical Interventions[edit | edit source]
For some individuals with refractory neuropathic pain, interventional treatments—which deliver medications to specific regions or modify particular brain structures—offer alternative therapy options.
Some of the interventional treatments include:
- Spinal cord stimulation: Requires the application of a monophasic square-wave pulse (at a frequency in the 30–100 Hz range) that results in paraesthesia of the painful part[11][12]
- Cortical stimulation: Using either invasive epidural or transcranial non-invasive procedures (such repetitive transcranial magnetic stimulation (TMS) and transcranial direct current stimulation), cortical stimulation entails stimulating the pre-central motor cortex below the motor threshold.
- Deep brain stimulation: The internal capsule, various nuclei in the sensory thalamus, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus, and anterior cingulate cortex are potential targets for pain relief in deep brain stimulation.
- Intrathecal treatments: In individuals with persistent pain that is severe and otherwise resistant to treatment, this offers a targeted drug delivery option to the painful part.[11]
Physical Therapy Management[edit | edit source]
Physical therapy modalities and rehabilitation techniques are important options and must be considered when pharmacotherapy alone is not sufficient [13].Physical therapy tackles the physical side of the inflammation, stiffness, and soreness with exercise, manipulation, and massage, but it also works to help the body heal itself by encouraging the production of the body's natural pain-relieving chemicals. This two-pronged approach is what helps make physical therapy so effective as a pain treatment.[14]
In addiction to that, exercise is useful to reorganise and sprouting the cortical body representation: the profound change in the neuromatrix related to chronic pain it is part of the central sensitisation [15][16].
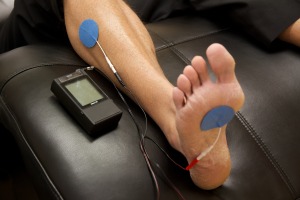
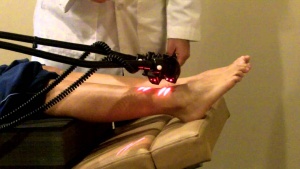
- TENS therapy has low quality of evidence for been effective in the treatment of painful peripheral neuropathy. Low level laser therapy could otherwise has positive effects on the control of analgesia for neuropathic pain [17][18].
- Neurostimulation techniques including transcranial magnetic stimulation (TMS) and cortical electrical stimulation (CES), spinal cord stimulation (SCS) and deep brain stimulation (DBS) have also been found effective in the treatment of neuropathic pain.
- Exercise and movement representation techniques (that is, treatments such as mirror therapy and motor imagery that use the observation and/or imagination of normal pain-free movements) have been suggested to be beneficial in neuropathic pain management. Recently, mirror therapy has used for not only patients with phantom limb pain, but also for patients with complex regional pain syndrome and strokes[19]. However the quality of evidence supporting these interventions for neuropathic pain is weak and needs further investigation.
- Exercise: exercising for just 30 minutes a day on at least three or four days a week will help you with chronic pain management by increasing:[14]Muscle Strength; Endurance; Stability in the joints; Flexibility in the muscles and joints. Keeping a consistent exercise routine will also help control pain. Regular therapeutic exercise will help you maintain the ability to move and function physically, rather than becoming disabled by your chronic pain.
- There are studies showing that exercise may be an important part of the treatment and prevention of neuropathic pain after chemotherapy. Although more information is required and detailed exercise prescriptions do not yet exist for patients receiving cancer treatment.[20] It has been also found that physical exercise, such as forced treadmill running and swimming, can sufficiently improve mechanical allodynia and heat hyperalgesia in animal models of neuropathic pain. [21]
Please watch the following video for relevant about neuropathic pain.
Management of Neuropathic Pain[22]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 PPM The Pathophysiology of Neuropathic Pain Available:https://www.practicalpainmanagement.com/pain/neuropathic/pathophysiology-neuropathic-pain (accessed 30.10.2021)
- ↑ 2.0 2.1 2.2 NICE. Neuropathic pain in adults: pharmacological management in non-specialist settings.Available: https://www.ncbi.nlm.nih.gov/books/NBK552848/(accessed 30.9.2021)
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annual Review of Pharmacology and Toxicology. 2020 Jan 6;60:257-74.
- ↑ Ochoa J, Torebjork HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–854.
- ↑ Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiological reviews. 2020 Nov 3.
- ↑ Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS . Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain, 2014; 155 (7): 1272-9
- ↑ Greening J, Lynn B. Minor peripheral nerve injuries an underestimated source of pain? Manual Therapy, 1998; 3: 187-194
- ↑ Gifford L. Acute low cervical nerve root conditions: symptom presentation and pathobiological reasoning. Manual Therapy, 2001; 6: 106-115
- ↑ Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion — a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38
- ↑ Collins KL, Russell HG, Schumacher PJ, et al. A review of current theories and treatments for phantom limb pain. J Clin Invest. 2018;128(6):2168-2176.
- ↑ 11.0 11.1 Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C. Neuropathic pain. Nature reviews Disease primers. 2017 Feb 16;3(1):1-9.
- ↑ Dones I, Levi V. Spinal cord stimulation for neuropathic pain: current trends and future applications. Brain sciences. 2018 Jul 24;8(8):138.
- ↑ Akyuz G, Kenis O. Physical therapy modalities and rehabilitation techniques in the management of neuropathic pain. Am J Phys Med Rehabil. 2014 Mar;93(3):253-9.
- ↑ 14.0 14.1 Physical Therapy for Pain Management.By Diana Rodriguez | Medically reviewed by Pat F. Bass III, MD, MPH
- ↑ Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001 Dec;65(12):1378-82.
- ↑ Trout KK. The neuromatrix theory of pain: implications for selected nonpharmacologic methods of pain relief for labor. J Midwifery Womens Health. 2004 Nov-Dec;49(6):482-8.
- ↑ Gibson W, Wand BM, O'Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. 2017 Sep 14;9(9):CD011976
- ↑ de Andrade AL, Bossini PS, Parizotto NA. Use of low level laser therapy to control neuropathic pain: A systematic review. J Photochem Photobiol B. 2016 Nov;164:36-42.
- ↑ Kim SY, Kim YY. Mirror therapy for phantom limb pain. Korean J Pain. 2012;25(4):272-274.
- ↑ Majithia N, Loprinzi CL, Smith TJ.New Practical Approaches to Chemotherapy-Induced Neuropathic Pain: Prevention, Assessment, and Treatment. Oncology (Williston Park). 2016 Nov 15;30(11)
- ↑ Kami K, Tajima F, Senba E. Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain. Anat Sci Int. 2016 Aug 2. [Epub ahead of print]
- ↑ Management of Neuropathic Pain. Available from: https://www.youtube.com/watch?v=hDu_WdRNDzo&t=796s