Neuropathic Pain: Difference between revisions
No edit summary |
No edit summary |
||
| Line 8: | Line 8: | ||
The International Association for the Study of Pain (2011) defines neuropathic pain as ‘pain caused by a lesion or disease of the somatosensory nervous system’. Central neuropathic pain is defined as ‘pain caused by a lesion or disease of the central somatosensory nervous system’, and peripheral neuropathic pain is defined as ‘pain caused by a lesion or disease of the peripheral somatosensory nervous system’. | The International Association for the Study of Pain (2011) defines neuropathic pain as ‘pain caused by a lesion or disease of the somatosensory nervous system’. Central neuropathic pain is defined as ‘pain caused by a lesion or disease of the central somatosensory nervous system’, and peripheral neuropathic pain is defined as ‘pain caused by a lesion or disease of the peripheral somatosensory nervous system’. | ||
Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies, symptoms and underlying mechanisms<ref>NICE. [https://www.ncbi.nlm.nih.gov/books/NBK552848/ Neuropathic pain in adults: pharmacological management in non-specialist settings].Available: https://www.ncbi.nlm.nih.gov/books/NBK552848/<nowiki/>(accessed 30.9.2021)</ref>. | Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies, symptoms and underlying mechanisms<ref name=":0">NICE. [https://www.ncbi.nlm.nih.gov/books/NBK552848/ Neuropathic pain in adults: pharmacological management in non-specialist settings].Available: https://www.ncbi.nlm.nih.gov/books/NBK552848/<nowiki/>(accessed 30.9.2021)</ref>. | ||
== Causes == | == Causes == | ||
Conditions frequently associated with neuropathic pain can be categorized into two major group, pain due to damage in the central nervous system and pain due to damage to the peripheral nervous system. | |||
Central nervous system causes include: | |||
* Cortical and sub-cortical strokes, traumatic spinal cord injuries, syringo-myelia and syringobulbia, trigeminal and glossopharyngeal neuralgias, neoplastic and other space-occupying lesions. | |||
Peripheral nervous system causes include: | |||
< | * Nerve compression/ entrapment neuropathies, ischemic neuropathy, peripheral polyneuropathies, plexopathies, nerve root compression, post-amputation stump and phantom limb pain, postherpetic neuralgia and cancer-related neuropathies. | ||
* Following a peripheral origin, central sensitization may develop as a result of ectopic neuronal activity in the [[Spinal cord anatomy|spinal cord]] dorsal horn, implying a potential autonomous pain-generating mechanism.<ref>Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS (2014). [https://pubmed.ncbi.nlm.nih.gov/24704366/ Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy.] Pain, 155 (7), 1272-9</ref> | |||
The mechanism underlying neuropathic pain, however, is not as clear. Several animal studies have shown that many mechanisms may be involved. | |||
First order neurons may increase their firing if they are partially damaged and increase the number of sodium channels. | |||
Ectopic discharges are a result of enhanced depolarization at certain sites in the fiber, leading to spontaneous pain and movement-related pain. | |||
Inhibitory circuits may be impaired at the level of the dorsal horn or brain stem (or both) allowing pain impulses to travel unopposed. | |||
In addition, there may be alterations in the central processing of pain when (due to [[Chronic Pain and the Brain|chronic pain]] and use of some drugs), second- and third-order neurons may develop a “memory” of pain and become sensitized. | |||
There is then heightened sensitivity of spinal neurons and reduced activation thresholds. <ref name=":0" /><br> | |||
<br> | |||
== Types of Neuropathic Pain == | == Types of Neuropathic Pain == | ||
| Line 65: | Line 54: | ||
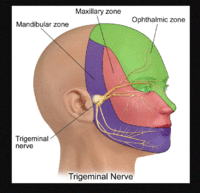
[[Trigeminal Neuralgia|Trigeminal neuralgia]] (TN) is a cause of severe pain in the face and jaw. Shocking, electric "lightening" pains typically precede dull aching pain. Trigeminal neuralgia usually affects only one side of the face. The exact cause of trigeminal neuralgia is unknown, but it develops where the trigeminal nerve is compressed, pinched, or irritated. | [[Trigeminal Neuralgia|Trigeminal neuralgia]] (TN) is a cause of severe pain in the face and jaw. Shocking, electric "lightening" pains typically precede dull aching pain. Trigeminal neuralgia usually affects only one side of the face. The exact cause of trigeminal neuralgia is unknown, but it develops where the trigeminal nerve is compressed, pinched, or irritated. | ||
[[Image:Trigeminal Branches.gif|200x220px]] | [[Image:Trigeminal Branches.gif|200x220px]]<br> | ||
<br> | |||
== Clinical Features == | == Clinical Features == | ||
Revision as of 01:53, 30 October 2021
Original Editor - Kerstin McPherson
Top Contributors - Andeela Hafeez, Melissa Coetsee, Kerstin McPherson, Vanessa Rhule, Riccardo Ugrin, Lucinda hampton, Kim Jackson, Admin, Evan Thomas, Chrysolite Jyothi Kommu, Kapil Narale, Temitope Olowoyeye, Jo Etherton, WikiSysop, Vidya Acharya, Lauren Lopez, Wendy Walker, Carina Therese Magtibay, Oyemi Sillo, Amanda Ager and 127.0.0.1
Definition[edit | edit source]
The International Association for the Study of Pain (2011) defines neuropathic pain as ‘pain caused by a lesion or disease of the somatosensory nervous system’. Central neuropathic pain is defined as ‘pain caused by a lesion or disease of the central somatosensory nervous system’, and peripheral neuropathic pain is defined as ‘pain caused by a lesion or disease of the peripheral somatosensory nervous system’.
Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies, symptoms and underlying mechanisms[1].
Causes[edit | edit source]
Conditions frequently associated with neuropathic pain can be categorized into two major group, pain due to damage in the central nervous system and pain due to damage to the peripheral nervous system.
Central nervous system causes include:
- Cortical and sub-cortical strokes, traumatic spinal cord injuries, syringo-myelia and syringobulbia, trigeminal and glossopharyngeal neuralgias, neoplastic and other space-occupying lesions.
Peripheral nervous system causes include:
- Nerve compression/ entrapment neuropathies, ischemic neuropathy, peripheral polyneuropathies, plexopathies, nerve root compression, post-amputation stump and phantom limb pain, postherpetic neuralgia and cancer-related neuropathies.
- Following a peripheral origin, central sensitization may develop as a result of ectopic neuronal activity in the spinal cord dorsal horn, implying a potential autonomous pain-generating mechanism.[2]
The mechanism underlying neuropathic pain, however, is not as clear. Several animal studies have shown that many mechanisms may be involved.
First order neurons may increase their firing if they are partially damaged and increase the number of sodium channels.
Ectopic discharges are a result of enhanced depolarization at certain sites in the fiber, leading to spontaneous pain and movement-related pain.
Inhibitory circuits may be impaired at the level of the dorsal horn or brain stem (or both) allowing pain impulses to travel unopposed.
In addition, there may be alterations in the central processing of pain when (due to chronic pain and use of some drugs), second- and third-order neurons may develop a “memory” of pain and become sensitized.
There is then heightened sensitivity of spinal neurons and reduced activation thresholds. [1]
Types of Neuropathic Pain[edit | edit source]
Entrapment Neuropathy[edit | edit source]
A trapped or pinched nerve at the neck, shoulder, elbow, wrist, hip, lower leg, or foot. Common examples of nerve entrapment include carpal tunnel syndrome, thoracic outlet syndrome (neck), or piriformis syndrome (hip).[3]
Peripheral Neuropathy[edit | edit source]
Peripheral neuropathy first develops in the longest nerves of the body in a "glove and stocking" distribution to the hands and feet. There are numerous causes of peripheral neuropathy, including certain hereditary conditions, viral diseases, liver or kidney failure, and toxins, as well as diseases such as diabetes, vascular disease, and rheumatoid conditions.[4] Peripheral neuropathy can be a motor, sensory or autonomic in nature.
Phantom Limb Pain[edit | edit source]
Phantom limb pain occurs in some people after the amputation of an arm or leg. Although the exact cause of phantom limb pain is unknown, it appears to result when the nerves and the brain send faulty signals to the limb as the circuitry attempts to "rewire" itself.[3]
Post Herpetic Neuralgia (PHN)[edit | edit source]
Post-herpetic neuralgia (PHN) is a type of nerve pain that can occur following a viral infection of herpes zoster "shingles" in the nervous system. Post-herpetic neuralgia aching or stabbing pain occurs in areas where the shingles rash developed. The skin in such areas may feel extra sensitive, especially in white-colored scars.[3]
Post Traumatic Neuropathy[edit | edit source]
Post Traumatic Neuropathy occurs after injury or medical procedures, such as surgery or injection. Nerve pain symptoms may arise at the injury site and nerve path.[3]
Trigeminal Neuralgia[edit | edit source]
Trigeminal neuralgia (TN) is a cause of severe pain in the face and jaw. Shocking, electric "lightening" pains typically precede dull aching pain. Trigeminal neuralgia usually affects only one side of the face. The exact cause of trigeminal neuralgia is unknown, but it develops where the trigeminal nerve is compressed, pinched, or irritated.
Clinical Features[edit | edit source]
Patients often find it difficult to describe the quality of NP; it is outside their previous experience of pain. Sensory loss may be mild and overshadowed by allodynia (all stimuli producing pain), hyperalgesia and hyperpathia (delayed perception, summation, and painful aftersensation). Rarely, (e.g. trigeminal neuralgia) there is no demonstrable sensory loss. There may be signs of sympathetic dysfunction and occasionally dystrophic changes.[5] The onset of pain may be delayed, the commonest example being central poststroke pain (thalamic), which may start months or years after the initiating stroke. Pain is often of mixed nociceptive and neuropathic types, for example, mechanical spinal pain with radiculopathy or myelopathy. It is not generally recognized that nociceptive spinal pain can radiate widely, mimicking a root distribution. It can be difficult to identify the dominant pain type and treat appropriately. Such patients require careful examination, imaging, and neurophysiological investigation.[5]
Pathophysiology[edit | edit source]
The pathophysiological properties that are responsible for NP can be broadly categorised into five groups: ectopic impulse generation in damaged primary afferent fibres, fibre interactions, central sensitisation, disinhibition (failure or reduction of normal inhibitory mechanisms), and plasticity (degenerative and regenerative changes associated with altered connectivity). [5]
- The mechanisms of NP are substantially different to those of nociceptive pain.
- Novel impulse generators develop at various sites, and these are not stimulus-dependent.
- In peripheral nerve, it has been shown that ectopic impulse generation (EIG) develops as a result of the expression of abnormal sodium channels. This can be modified by neurotrophic growth factors (a potential target for new treatments).[5]
- Abnormal chemical sensitivities develop in damaged primary sensory neurons, notably to catecholamines. Whilst this can be readily demonstrated in experimental preparations, the clinical relevance remains uncertain.
- Degenerative and then regenerative changes in the spinal cord may lead to aberrant connectivity, and possibly a permanently reorganised, irreversible state.[5]
- Damage at one level in the nervous system may lead to secondary pathophysiological changes at more rostral levels. This has important implications when targeting treatments for NP.
Assessment[edit | edit source]
Screening questionnaires are suitable for identifying potential patients with neuropathic pain, but further validation of them is needed for epidemiological purposes. Clinical examination, including accurate sensory examination, is the basis of neuropathic pain diagnosis. For more accurate sensory profiling, quantitative sensory testing is recommended for selected cases in clinic, including the diagnosis of small fiber neuropathies and for research purposes.[7]
Step 1. A clinical history of disease or lesion of the somatosensory system suggests a possible diagnosis of neuropathic pain
Step 2. Confirmation by either clinically reproducible signs or investigations would suggest a probable diagnosis of neuropathic pain
Step 3. If the history, clinical examination, and investigations are positive, this would support a definite diagnosis of neuropathic pain[8]
Sensory examination - light touch, temperature, painful stimulus, vibration, and proprioception. Motor testing tone, strength, reflexes, and coordination. Look for autonomic changes in colour, temperature, sweating and swelling.
Examination of a Patient with Peripheral Neuropathic Pain shows a real patient with multiple mononeuropathy due to isolated peripheral nervous system vasculitis. He is suffering from neuropathic pain in his left hand and both legs. Functional assessment and sensory and motor examination of both upper and lower limbs is demonstrated. DN4 questionnaire assists with neuropathic pain assessment.
Use when neuropathic pain is suspected[9]
Management[edit | edit source]
Unfortunately, neuropathic pain often responds poorly to standard pain treatments and occasionally may get worse instead of better over time. For some people, it can lead to serious disability. A multidisciplinary approach that combines therapies, however, can be a very effective way to provide relief from neuropathic pain.[10]
Other kinds of treatments can also help with neuropathic pain. Some of these include:
- Physical therapy
- Counselling
- Relaxation therapy
- Massage therapy
- Acupuncture
Medical management[edit | edit source]
Anticonvulsant and antidepressant drugs, for example, pregabalin, gabapentin, and amitriptyline may work to reduce symptoms in most cases. Some neuropathic pain studies suggest the use of non-steroidal anti-inflammatory drugs, such as Aleve or Motrin, may ease pain. Additionally, some people may require a stronger painkiller, such as those containing morphine and can be used if the patient does not respond to other therapies.
If another condition, such as diabetes, is involved, better management of that disorder may alleviate the pain. Effective management of the condition can also help prevent further nerve damage[11].In cases that are difficult to treat, a pain specialist may use an invasive or implantable device to effectively manage the pain. Electrical stimulation of the nerves involved in neuropathic pain may significantly control the pain symptoms.[10]
Physical therapy management[edit | edit source]
TENS is effective in the treatment of painful peripheral neuropathy, and very low level laser therapy has been shown to be effective in patients with neuropathic pain[12].
Neurostimulation techniques including transcranial magnetic stimulation (TMS) and cortical electrical stimulation (CES), spinal cord stimulation (SCS) and deep brain stimulation (DBS) have also been found effective in the treatment of neuropathic pain. In general, deep heating agents like ultrasound and short wave diathermy are not recommended in the treatment of neuropathic pain. [12]
Exercising for just 30 minutes a day on at least three or four days a week will help you with chronic pain management by increasing:[13]
- Muscle Strength Testing
- Endurance
- Stability in the joints
- Flexibility in the muscles and joints
Keeping a consistent exercise routine will also help control pain. Regular therapeutic exercise will help you maintain the ability to move and function physically, rather than becoming disabled by your chronic pain.
There are studies showing that exercise may be an important part of the treatment and prevention of neuropathic pain after chemotherapy. Although more information is required and detailed exercise prescriptions do not yet exist for patients receiving cancer treatment.[14] It has been also found that physical exercise, such as forced treadmill running and swimming, can sufficiently improve mechanical allodynia and heat hyperalgesia in animal models of neuropathic pain. [15]
Although there is not much research on this subject, some article points out that low level laser therapy could help in the treatment of neuropathic pain.[16]
Physical therapy tackles the physical side of the inflammation, stiffness, and soreness with exercise, manipulation, and massage, but it also works to help the body heal itself by encouraging the production of the body's natural pain-relieving chemicals. This two-pronged approach is what helps make physical therapy so effective as a pain treatment.[13]
LINKS
References[edit | edit source]
- ↑ 1.0 1.1 NICE. Neuropathic pain in adults: pharmacological management in non-specialist settings.Available: https://www.ncbi.nlm.nih.gov/books/NBK552848/(accessed 30.9.2021)
- ↑ Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrøm JB, Jensen TS (2014). Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain, 155 (7), 1272-9
- ↑ 3.0 3.1 3.2 3.3 Neuropathy-Nerve pain-Dr. Mary Car-Blanchard, OTD/OTR/L and the following editorial advisors: Steve Meadows, MD, Ernie F. Soto, DDS, Ronald J. Glatzer, MD, Jonathan Rosenberg, MD, Christopher M. Nolte, MD, David Ap
- ↑ Neuropathy-Nerve pain-Dr. Mary Car-Blanchard, OTD/OTR/L and the following editorial advisors: Steve Meadows, MD, Ernie F. Soto, DDS, Ronald J. Glatzer, MD, Jonathan Rosenberg, MD, Christopher M. Nolte, MD, David Ap
- ↑ 5.0 5.1 5.2 5.3 5.4 http://www.acnr.co.uk/pdfs/volume3issue2/v3i2reviewart1.pdf
- ↑ Transdermal Therapeutics Inc. Neuropathic Pain. Available from: https://www.youtube.com/watch?v=CUH7RTus880
- ↑ NeuPSIG guidelines on neuropathic pain assessment Maija Haanpää , Nadine Attal, Miroslav Backonja, Ralf Baron, Michael Bennett, Didier Bouhassira, Giorgio Cruccu, Per Hansson, Jennifer Haythornthwaite, Gian Domenico Iannetti, Troels S Jensen, Timo Kauppila, Turo J Nurmikko, Andew S C Rice, Michael Rowbotham, Jordi Serra, Claudia Sommer, Blair H Smith, Rolf-Detlef Treede.PMID: 20851519.DOI: 10.1016/j.pain.2010.07.031
- ↑ Adapted from Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70:1630–5
- ↑ Bouhassira D, Attal N, Alchaar H, et al. "Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4)." P
- ↑ 10.0 10.1 http://www.webmd.com/pain-management/guide/neuropathic-pain
- ↑ http://www.webmd.com/pain-management/guide/neuropathic-pain
- ↑ 12.0 12.1 http://www.omicsonline.org/physical-therapy-modalities-and-rehabilitation-techniques-in-the-treatment-of-neuropathic-pain-jpmr.1000124.pdf
- ↑ 13.0 13.1 Physical Therapy for Pain Management.By Diana Rodriguez | Medically reviewed by Pat F. Bass III, MD, MPH
- ↑ Majithia N, Loprinzi CL, Smith TJ.New Practical Approaches to Chemotherapy-Induced Neuropathic Pain: Prevention, Assessment, and Treatment. Oncology (Williston Park). 2016 Nov 15;30(11)
- ↑ Kami K, Tajima F, Senba E. Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain. Anat Sci Int. 2016 Aug 2. [Epub ahead of print]
- ↑ Andrade AL, Bossini PS, Parizotto NA. Use of low level laser therapy to control neuropathic pain: A systematic review. J Photochem Photobiol B. 2016 Nov;164:36-42.
- ↑ Victoria Pain Specialists. Neuropathic Pain.