Cauda Equina Syndrome
Original Editor - Laurie Fiegle and Tabitha Korona
Top Contributors - Laura Finucane, Tabitha Korona, Scott Buxton, Admin, Laura Ritchie, Thibaut Seys, Laurie Fiegle, Jolien Wauters, Kim Jackson, Evan Thomas, Rachael Lowe, Margo De Mesmaeker, Naomi O'Reilly, WikiSysop, Simisola Ajeyalemi, Claire Knott, Lucinda hampton, Garima Gedamkar, Kai A. Sigel, 127.0.0.1, Karen Wilson, Tony Lowe, Candace Goh, Shaimaa Eldib, Jess Bell, Olajumoke Ogunleye and Saud Alghamdi
Definition[edit | edit source]
Cauda equina syndrome (CES) is a rare but serious neurological condition affecting the bundle of nerve roots at the lower end of the spinal cord. The CE provides innervation to the lower limbs, and sphincter,controls the function of the bladder and distal bowel and sensation to the skin around the bottom and back passage[1].
CES occurs when the nerves below the spinal cord are compressed causing compromise to the bladder and bowel. The most common cause of CES is a prolapse of a lumbar disc but other conditions such as metastatic spinal cord compression can also cause CES[1].
There is no agreed definition of CES but the British Association of Spinal Surgeons (BASS) present a definition that is useful in clinical practice;
'A patient presenting with acute back pain and/or leg pain...... with a suggestion of a disturbance of their bladder or bowel function and/or saddle sensory disturbance should be suspected of having a CES. Most of these patients will not have critical compression of the cauda equina. However, in the absence of reliably predictive symptoms and signs, there should be a low threshold for investigation with an emergency scan'[2].
Classification[edit | edit source]
4 groups of patients have been classified according to their presentation :[3]
CESS- Suspected
Patients who do not have CES symptoms but who may go on to develop CES. It is important that patients understand the gravity of the condition and the importance of the time frame to seeking urgent medical attention. The use of a credit card style patient information leaflet or a leaflet explaining what to look for and what to do should they develop symptoms is recommended.
CESI- Incomplete
Patients who present with urinary difficulties with a neurogenic origin, including loss of desire to void, poor stream, needing to strain to empty their bladder, and loss of urinary sensation. These patients could develop CESR and are a medical emergency and should be decompressed urgently.
CESR -Retention
Patients who present with painless urinary retention and overflow incontinence; the bladder is no longer under executive control.
Surgical intervention is necessary and should be carried out as soon as practically possible.
CESC-Complete
Patients who have objective loss of the cauda equina function, absent perineal sensation, a loose anus and paralysed bladder and bowel.
Clinically Relevant Anatomy[edit | edit source]
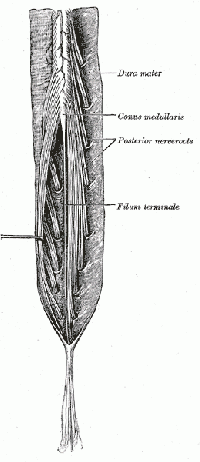
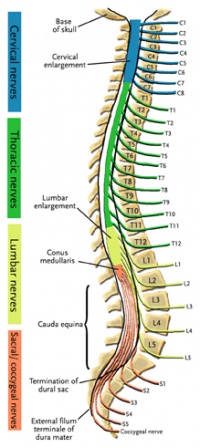
The central nervous system (CNS) includes the brain and spinal cord. The spinal cord allows transmission of signals to and from the brain to allow for movement, sensation, and visceral function. Nerves exiting the spinal cord are divided into sections based on their location of exit; for example, cervical nerves exit the cervical spine and thoracic nerves exit the thoracic spine. The conus medullaris is the distal end of the spinal cord and is usually located at the L1-L2 vertebral level[5].
Nerve roots continue to down the vertebral column after the conus medullaris and are referred to as the cauda equina. The cauda equina includes nerves from lumbar, sacral and coccygeal origins. Being a bundle of nerves, the cauda equina is named for its resemblance to a horse's tail.
The spinal cord extends from the medulla oblongata to the level of T12-L1 where it terminates as the conus medullaris[6]. The cauda equina consists of nerve roots distal to the conus medullaris. These have both a dorsal and ventral root. Each root has specific function. The ventral root provides motor fibers for the efferent pathway and sympathetic fibers[6][7]. The dorsal root is composed of afferent fibers for the transmission of sensation. Orientation within the cauda equina is unique and specific[7]. The lower lumbar and all the sacral nerves come together in the cauda equina region. The functions of those nerves are[6]:
- Sensory innervation to the saddle area
- Voluntary control of the external anal and urinary sphincters
- Sensory and motor fibres to the lower limbs.
Epidemiology/Etiology[edit | edit source]
CES occurs as a consequence of compression of the cauda equina and can be caused by a number of pathologies. The prevalence among the general population has been estimated between 1:100 000 and 1:33 000. The most common cause of CES is herniation of a lumbar intervertebral disc[5] and accounts for 2% of all herniated lumbar discs.[8] It commonly affects the discs at the L4/5 and L5/S1 level . However disc prolapse at any lumbar level can cause CES. Patients may be predisposed to CES if they have a congenitally narrow spinal canal or have acquired spinal stenosis. [9]The prevalence among patients with low back pain is approximately four in 10 000[10].
CES affects males and females equally and can occur at any age but primarily in adulthood.
Other pathologies which can cause CES include spinal stenosis, haematoma, trauma [11] tumour, infection, fracture and inflammatory conditions. [1][12][13]
Other rare causes such as abdominal aortic dissection, and complications after surgery, anesthetic procedures, spinal manipulation or epidural injections are possible causes of CES[1].
Clinical Presentation[edit | edit source]
5 characteristic features of CES are consistently described in the literature and should form the basis of questions related to diagnosis[3];
· bilateral neurogenic sciatica
· reduced perineal sensation
· altered bladder function leading to painless retention
· loss of anal tone
· loss of sexual function
Pain associated with the back and/ or leg symptoms maybe present.
Bladder dysfunction is the most commonly reported symptom and can range from increased frequency , difficulty in micturation, incontinence and retention.
Bowel dysfunction includes incontinence, inability to control motions, inability to feel when the bowel is full and consequently overflow.
Since the cauda equina nerve roots supply most of the lower extremities (including the pelvic region) sensory and motor innervations, cauda equina syndrome results in multiple motor and sensory signs. The most common signs and symptoms include bilateral sciatica, saddle region anesthesia, loss of bowel and bladder control, bilateral foot weakness, quadriceps weakness, and severe back pain. Other signs and symptoms include decreased sensation between the legs, sexual and genitourinary dysfunction (with overflow, incontinence or retention)[14] buttocks, or feet[15].
Differential Diagnoses[edit | edit source]
- Conus medullaris syndrome
- Spinal tumor
- Abscesses[7]
- Central or centerolateral disk prolapsed[17]
- Space-occupying lesions that compress nerve roots have been described as causes of CES.
- Canal stenosis [12]
- Spinal Anesthesia
- Neoplasm [13]
- Ischemia
- Infections
- Inflammatory conditions
- Osteomyelitis
Diagnostic Procedures[edit | edit source]
The diagnosis of cauda equina syndrome generally is possible based on medical history and physical examination findings. Radiologic and laboratory studies are used to confirm the diagnosis and for localizing the site of the pathology and the underlying cause.[16]
A physical exam is necessary to assess the strength, reflexes sensation, stability (=a posture / movement where an ideal distribution of body mass is reached. This provides the body with conditions for normal function during stationary positions or movements (sitting ⇒ stand, walk, walk, ...)) , alignment and motion. The physical exam is an essential part of any doctor's visit. Surprisingly, though, there are no absolutes (= assurances that signs are pointing in the direction of CES) in a routine physical examination. If there is a loss of function from the muscles, strength tests are used to confirm.
Electromyography (EMG) may show evidence of acute denervation and could also help in predicting prognosis and monitoring recovery. Performing an EMG of the bilateral external anal sphincter muscles is recommended.[18]
If cauda equina syndrome is expected, medical referral is a necessity to decrease the possibility of permanent damage. Diagnostic procedures used to confirm cauda equina syndrome may include an MRI or myelogram[19]. For diagnosis of cauda equine syndrome, one or more of the following must be present[17][20]
- Bladder and/or bowel dysfunction
- Reduced sensation in saddle area
- Sexual dysfunction
- Possible neurological deficit in the lower limb (motor/sensory loss, reflex change)
While MRI, coupled with patient history and examination, remains the diagnostic gold standard, it comes at a high cost with many patients demonstrating no concordant pathology.[21]
Examination[edit | edit source]
A patient who complains of severe leg weakness, numbness in the genital area, or loss of bladder or bowel function will undergo an MRI scan to reveal the extent to which the herniation is compressing the spinal nerves. The doctor may also order a CT scan or a myelogram.[22][23]
Pain often is localized to the low back; local tenderness to palpation or percussion may be present. Pain in the legs (or radiating to the legs) is characteristic of cauda equina syndrome. Radicular pain is a common presentation in patients with cauda equina syndrome, usually in association with radicular sensory loss (saddle anesthesia), asymmetric paraplegia with loss of tendon reflexes, muscle atrophy, and bladder dysfunction. The presentation is somewhat similar to and is often confused with conus and epiconus lesions. Reflex abnormalities may be present; they typically include loss or diminution of reflexes. Hyperactive reflexes may signal spinal cord involvement and exclude the diagnosis of cauda equina syndrome. Sensory abnormality may be present in the perineal area or lower extremities. Light touch in the perineal area should be tested. Anesthetic areas may show skin breakdown.
Muscle weakness may be present in muscles supplied by affected roots. Muscle wasting may occur in chronic cauda equina syndrome.
Poor anal sphincter tone is characteristic of cauda equina syndrome. Babinski sign or other signs of upper motor neuron involvement suggest a diagnosis other than cauda equina syndrome, possibly spinal cord compression.
Physical examination for cauda equina or conus medullaris syndromes would be incomplete without tests for sensation of the saddle and perineal areas, bulbocavernosus reflex, cremasteric reflex, and anal sphincter tone, findings for all of which are likely to be abnormal.
Muscle strength of the following muscles should be tested to determine the level of lesion:
- L2 - Hip flexors (iliopsoas)
- L3 - Knee extensors (quadriceps)
- L4 - Ankle dorsiflexors (tibialis anterior)
- L5 - Big toe extensors (extensor hallucis longus)
- S1 - Ankle plantar flexors (gastrocnemius/soleus)
In defining impairments associated with a spinal cord lesion, the American Spinal Cord Injury Association (ASIA) impairment scale is used in determining the level and extent of injury. This scale should also be used in defining the extent of conus medullaris syndrome/cauda equina syndrome. The scale is as follows:
- A - Complete; no sensory or motor function preserved in sacral segments S4-S5
- B - Incomplete; sensory, but not motor, function preserved below the neurologic level and extends through sacral segments S4-S5
- C - Incomplete; motor function preserved below the neurologic level, and the majority of key muscles below the neurologic level have a muscle grade less than 3
- D - Incomplete; motor function preserved below the neurologic level, and the majority of key muscles below the neurologic level have a muscle grade greater than or equal to 3
- E - Normal; sensory and motor function normal [24]
Medical Management[edit | edit source]
Any patient with urinary dysfunction must be studied on an emergent basis, particularly if the patient has suffered an acute change. The burden to the patient in the setting of missed or delayed diagnosis of cauda equina syndrome may be devastating because the patients can lose bowel, bladder, and sexual function, which can negatively affect patient health and quality of life. By operating <48 hours of cauda equina syndrome onset and communicating with the patient the potential outcome, a greater chance exists that one can reduce the considerable financial burden to institutions and have a less adverse outcome in court.[26] [2A]
Once CES is diagnosed, emergent surgical decompression is recommended to avoid potential permanent neurological damage[10].[1A]
Time from symptom onset to surgery is not correlated with persistence of major symptoms. [8] [2A]
The emergent surgical decompression must take place as soon as possible in order to reduce or eliminate pressure on the nerve. It is recommended to perform the decompression within 24-48 hours after the appearance of the first symptoms of compression so that there is a maximum potential for improvement of sensory and motor deficits as well as bladder and bowel functioning[27]. [1A]
The role of surgery is to relieve pressure from the nerves in the cauda equina region and to remove the offending elements[27]. [1A]
Surgical strategy is usually focused on the underlying causes. Generally, spine posterior decompression is often adequate - unless there is a lesion such as vertebrae destruction, neoplasm or large abscess in the anterior spine. Multiple surgical approaches of decompression are recommended such as discectomy, microdiscectomy, microscopic decompression, fenestrations, laminectomy, hemisemilaminotomy, distraction laminoplasty, multilevel laminectomies, neurolysis of CE, and intradural exploration of the nerve roots[10].[1A]
The surgical decompression takes away the cause of pain but most individuals still have complaints afterwards:
- Bladder and bowel dysfunction
- Muscle weakness or paralysis in lower extremities - Walking disorders[17] [1A]
Most recovery takes place in the first year after the operation; however, there can be recovery up to the third year post-operation. After this time period, recovery is very minimal[28]. [3A]
When there is a CES secondary to L5 giant cell tumour (GCT), these patiënts can be treated with denosumab and without surgery. The usage of denosumab allows potential neurological recovery without any surgical intervention. If surgery is not contraindicated, more time is obtained to prepare the patient preoperatively to attain safer surgery and to achieve complete tumour clearance.[29] [4]
Physical Therapy management[edit | edit source]
The ultimate goals of physical management are to ensure maximum neurological recovery and independence, a pain-free and flexible spine, maintenance of mobility and strength in lower limbs, of core strength, improvement of standing and walking function, improvement of bladder, bowel and sexual function, improvement of endurance and safe functioning of the various systems of the body with minimal or no inconvenience to patients and prevention or minimization of complications[17]. [1A] It is equally important for patients to regain assertiveness, take control of their own lives, and return to activities of their choice. The importance of on-going support to maintain health and independence following discharge should be strongly emphasized[27][28].[1A][3A]
Locomotion training as a therapeutic exercise was initially recognized in spinal cord injury patients, beginning with Body-weight Supported Treadmill Training (BWSTT) and Knee Ankle Foot Orthosis (KAFOs) personalized in soft-cast for the stabilization of the limbs. They have experienced Patterned Electrical Stimulation (PES) assisted isometric exercise to prevent limb muscle atrophy. It is known that PES-assisted isometric exercise reduces the degree of lower limb muscle atrophy in individuals with recent motor complete spinal cord injury, but not to the same extent as a comparable program of FES assisted exercise[30].[1B]
The principles of using electrical stimulation of peripheral nerves or nerve roots for restoring useful bladder, bowel, and sexual function after damage or disease of the central nervous system are described. Activation of somatic or parasympathetic efferent nerves can produce contraction of striated or smooth muscle in the bladder, rectum, and sphincters. Activation of afferent nerves can produce reflex activation of somatic muscle and reflex inhibition or activation of smooth muscle in these organs. In clinical practice these techniques have been used to produce effective emptying of the bladder and bowel in patients with spinal cord injury and to improve continence of urine and feces[31].[1A]
The use of manual therapy in conjunction with exercise is of potential benefit for patients suffering from low back pain. Utilization of manual therapy in a management program is associated with improvements in pain and disability. It is noteworthy to mention, however, that the manual therapy used in these studies was not of uniform technique nor applied only to one region. The techniques used in these studies were varied, and included both thrust and non-thrust manipulation/mobilization. Successful results were reported with techniques described as follows: flexion distraction manipulations, sidelying lumbar rotation thrust, posterior-to-anterior mobilizations, sidelying translatoric side bending manipulations, thoracic thrusts and neural mobilizations[32].[1A]
Individualized exercises often include components of unweighted walking or cycling, spinal mobility and lumbar flexion exercises, hip mobility exercises, hip strengthening, and core strengthening[32].[1A]
After surgery, there is also a need for recovery. Adjuvant radiotherapy and chemotherapy were performed in a case and showed 1-year after the operation no more problems. The craniospinal MRI was normal, and no recurrence was observed.[33] [5]
Site-specific radiotherapy was initiated with Bevacizumab in combination with vinorelbine. Upon completion of radiotherapy a radiographic response in both the brain and spine was confirmed. Also, the headaches, diplopia and incontinence significantly improved, and the patient was able to resume walking without assistance and became independent in her daily activities. [34] [5]
Key Evidence[edit | edit source]
Cauda equina syndrome is rare and is estimated to account for fewer than 1 in 2000 of patients with severe low back pain[17]
The annual incidence rate of cauda equina lesions has been estimated at 3.4 per million and the period prevalence at 8.9 per 100,000[5]
It is a disease of low incidence in the population, at around 1 case per 33000 to 1 case per 100000 inhabitants.[12]
It’s accounting for a reported incidence of 1-5% of spinal pathology in the literature[35].
Resources[edit | edit source]
• http://orthoinfo.aaos.org/topic.cfm?topic=A00362
• http://www.spine-health.com/conditions/lower-back-pain/cauda-equina-syndrome
• http://mayfieldclinic.com/PE-CaudaEquina.htm
• https://www.spineuniverse.com/conditions/back-pain/low-back-pain/cauda-equina-syndrome
• http://www.emedicinehealth.com/cauda_equina_syndrome/article_em.htm
• http://www.livestrong.com/article/228836-bowel-bladder-complications-from-a-lumbar-herniated-disc/
• http://www.aans.org/Patient%20Information/Conditions%20and%20Treatments/Cauda%20Equina%20Syndrome.aspx
• http://www.medicinenet.com/cauda_equina_syndrome/article.htm
• http://www.caudaequina.org/definition.html
• https://radiopaedia.org/articles/cauda-equina-syndrome
• http://www.emdocs.net/cauda-equina-syndrome/
• http://www.oapublishinglondon.com/article/1456#
• http://emedicine.medscape.com/article/1148690-clinical - b3
Clinical Bottom Line[edit | edit source]
Cauda equina syndrome is an uncommon but serious neurological condition affecting the bundle of nerve roots at the lower end of the spinal cord. It is due to a nerve compression that an acute loss of function of the lumbar plexus occurs which stops the sensation and movement. Cauda equina syndrome can be caused by a number of etiologies but the most common relate to compression of the cauda equina such as a herniated lumbosacral disc, spinal stenosis, hematoma, trauma, and spinal neoplasm. Ruptured disc, tumor, or fracture can also lead to cauda equina syndrome. Non-compressive causes include ischemia, infection, and inflammatory conditions. But there are a portion of cases that can’t be identified. The diagnosis of cauda equina syndrome generally is possible on the basis of medical history and physical examination findings. Diagnostic procedures used to confirm cauda equina syndrome may include an MRI or myelogram. Once CES is diagnosed, emergent surgical decompression is recommended to avoid potential permanent neurological damage. The emergent surgical decompression must take place as soon as possible in order to reduce or eliminate pressure on the nerve. They have experienced Patterned Electrical Stimulation (PES) assisted isometric exercise to prevent limb muscle atrophy. They use electrical stimulation of peripheral nerves or nerve roots for restoring useful bladder, bowel, and sexual function after damage or disease of the central nervous system. Utilization of manual therapy in a management program is associated with improvements in pain and disability. Individualized exercises often include components of unweighted walking or cycling, spinal mobility and lumbar flexion exercises, hip mobility exercises, hip strengthening, and core strengthening.
Recent Related Research (from Pubmed)[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 Fraser s, Roberts L, Murphy E. Cauda Equina Syndrome: A Literature Review of Its Definition and Clinical Presentation. Archives of Physical Medicine and Rehabilitation 2009 90(11), pp.1964–1968.
- ↑ Germon T, Ahuja,S, Casey A, Rai A. British Association of Spine Surgeons standards of care for cauda equina syndrome. The Spine Journal 2015 15 (3), pS2-S4.
- ↑ 3.0 3.1 Todd, N V; Dickson, R A . (2016). Standards of care in cauda equina syndrome. British Journal of Neurosurgery. 30 (5), p518-522.
- ↑ CES UK. Presentation - A Neurological Perspective of Cauda Equina Syndrome . Available from: http://www.youtube.com/watch?v=MLnY_esmmhE [last accessed 20/04/14]
- ↑ 5.0 5.1 5.2 Boek: Dutton, M. (2008). Orthopaedic: Examination, evaluation, and intervention (2nd ed.). New York: The McGraw-Hill Companies, Inc.
- ↑ 6.0 6.1 6.2 John McNamee, Peter Flynn, [...], and Barry Kelly, Imaging in Cauda Equina Syndrome – A Pictorial Review, The ulster Medical Journal, Januarie 1, 2013. Pictorial review. [5]
- ↑ 7.0 7.1 7.2 7.3 Alex Gitelman, MD, Shuriz Hishmeh, MD, Brian N. Morelli, Cauda Equina Syndrome: A Comprehensive Review, The American Journal of Orthopedics®, 2008. [3A]
- ↑ 8.0 8.1 Bydon, Mohamad, et al. "Time to Surgery and Outcomes in Cauda Equina Syndrome: An Analysis of 45 Cases." World neurosurgery 87 (2016):110-115.[Level Of Evidence: 2A]
- ↑ Dhokia R, Eames N. Cauda Equina Syndrome: A review of the current position. Hard Tissue 2014 Apr 18;3(1):7. [Level Of Evidence 3A]
- ↑ 10.0 10.1 10.2 MA Bin, WU Hong, JIA Lian-shun, YUAN Wen, SHI Guo-dong and SHI Jian-gang, Cauda equina syndrome: a review of clinical progress, Chinese Medical Journal, 2009 [1A]
- ↑ Song H. et al. Early surgery predicts a better prognosis of urinary function in cauda equine syndrome with retention: a systematic review and meta-analysis. Int J Clin Exp Med 2016;9(2):544-551. [Level Of Evidence: 1A]
- ↑ 12.0 12.1 12.2 Fuso FAF, Dias ALN, Letaif OB, Cristante AF, Marcon RM, Barros Filho TEP. Epidemiological study of cauda equina syndrome. Acta Ortop Bras. [online]. 2013;21(3):159- 62. [Level Of Evidence: 4]
- ↑ 13.0 13.1 13.2 Panos G. Et al. Differential diagnosis and treatment of acute cauda equina syndrome in the human immunodeficiency virus positive patient: a case report and review of the literature. Journal of medical case report 2016; 10:165. [Level Of Evidence: 3A]
- ↑ Bang J. et al. Missed cauda equina syndrome after burst fracture of the lumbar spine. Korean journal of neurotrauma 2015; 11(2): 175-179. [Level Of Evidence: 3B]
- ↑ Shapiro S. Medical Realities of Cauda Equina Syndrome Secondary to Lumbar Disc Herniation. SPINE 2000;25:3, 348-52
- ↑ 16.0 16.1 Ahad A. Et al. The accurancy of clinical symptoms in detecting cauda equina syndrome in patients undergoing acute MRI of the spine. The neuroradiology journal. 2015; 28(4): 438-442. [Level Of Evidence: 2B]
- ↑ 17.0 17.1 17.2 17.3 17.4 Stuart Fraser, Lisa Roberts, Eve Murphy, Cauda Equina Syndrome: A Literature Review of Its Definition and Clinical Presentation, Archives of Physical Medicine and Rehabilitation, Volume 90, Issue 11, Pages 1964-1968, November 2009 [1A]
- ↑ Podnar S. Utility of sphincter electromyography and sacral reflex studies in women with cauda equina lesions. Neurourology and urodynamics. 2014; 33:426-430 [Level Of Evidence 4]
- ↑ Ogilvie J. Complications in Spondylolisthesis Surgery. SPINE 2005;30:65 S97-S101
- ↑ Shi J. et al. Clinical classification of cauda equina syndrome for proper treatment. Acta orthop 2010 Jun;81(3):391-5. [1B]
- ↑ Fairbank J. Et al. Does patient history and physical examination predict MRI proven cauda equina syndrome? Evidence based spine care journal 2011; 2(4): 27-33. [Level Of Evidence: 1]
- ↑ Tien Chau AM, Xu LL, Pelzer NR, Gragnaniello C. Timing of Surgical Intervention in Cauda Equina Syndrome - a Systematic Critical Review. World Neurosurg. Nov 12, 2013 [ePub]. [Level Of Evidence 3A]
- ↑ Lavy C, James A, Wilson-MacDonald J, Fairbank J. Cauda equina syndrome. BMJ 338:b936, 2009. [Level Of Evidence 2A]
- ↑ Segun Toyin Dawodu et al., Cauda Equina and Conus Medullaris Syndromes Clinical Presentation; May 06, 2016, Medscape 1148690. [Level Of Evidence 1A]
- ↑ Robert Hacker. Microsurgery for disc herniation with Cauda Equina Syndrome. Available from: http://www.youtube.com/watch?v=kbcXBk3sqrU [last accessed 20/04/14]
- ↑ Daniels, Eldra W., et al. "Review of medicolegal cases for cauda equina syndrome: what factors lead to an adverse outcome for the provider?." Orthopedics 35.3 (2012): e414-e419.[Level Of Evidence: 2A]
- ↑ 27.0 27.1 27.2 A. Gardner, E. Gardner and T. Morley, Cauda equina syndrome: a review of the current clinical and medico-legal position, Eur Spine J. 2011 May; 20(5): 690–697. [1A]
- ↑ 28.0 28.1 Wagih E.M., Management of Traumatic Spinal Cord Injuries: current standard of care revisited, ACNR, Volume 10 Nr.1, March/April 2010 [3A]
- ↑ Randhawa, Simret Singh, et al. "Neurological Recovery in Two Patients with Cauda Equina Syndrome Secondary to L5 Lumbar Spine Giant Cell Tumour after Treatment with Denosumab without Surgery." Asian Spine Journal 10.5 (2016): 945-949.[Level Of Evidence: 4]
- ↑ Emiliana B., Agostino Z., Cristina M., Epidemiology and clinical management of Conus-Cauda Syndrome and flaccid paraplegia in Friuli Venezia Giulia: Data of the Spinal Unit of Udine, Basic Applied Myology 19 (4): 163-167, 2009 [1B]
- ↑ Creasey GH., Craggs MD., Functional electrical stimulation for bladder, bowel and sexual function, Spinal Cord Injuries, 2012;109:247-57 [1A]
- ↑ 32.0 32.1 Karen M.B., Julie M.W., Timothy W.F., Lumbar spinal stenosis-diagnosis and management of the aging spine, Manual Therapy 16, 2011 (308-317) [1A]
- ↑ Kotil, Kadir, Bekir Mahmut Kilinc, and Turgay Bilge. "Spinal metastasis of occult lung carcinoma causing cauda equina syndrome." Journal of clinical neuroscience 14.4 (2007): 372-375.[Level Of Evidence 5]
- ↑ Le Rhun, Emilie, et al. "Prolonged Response and Restoration of Functional Independence with Bevacizumab plus Vinorelbine as Third-Line Treatment for Breast Cancer-Related Leptomeningeal Metastases." Case reports in oncology 8.1 (2015): 72-77.[Level Of Evidence 5]
- ↑ J. G. Kennedy, K. E. Soffe, A. McGrath, M. M. Stephens, Predictors of outcome in cauda equina syndrome, Eur Spine J, 12 april 1999. [2B]