Noninvasive Brain Stimulation (NIBS)
Original Editor - Angeliki Chorti
Top Contributors - Angeliki Chorti
Introduction[edit | edit source]
Noninvasive Brain Stimulation (NIBS) is an umbrella term for any form of neurostimulation in vivo, that is not using surgery or anaesthesia in a target area of the brain. [1] NIBS has received great attention from clinicians and researchers, because of its potential as a neurophysiologic diagnostic tool for the brain and because it can provide non-pharmacological noninvasive neuromodulatory treatments for diseases of the brain. [2]
History of Brain Stimulation[edit | edit source]
The use of electricity for brain healing has been long documented; first, in stone carvings from the Fifth Dynasty of Egypt in which an electric fish was used to treat pain; and later, during the time of Socrates, when electric fish were used to treat headaches and arthritis. [2]
In 1831, Michael Faraday 's discovery of electromagnetic induction (i.e. a varying magnetic field induces electrical current in a conductor placed within the field) contributed to understanding this phenomenon. Besides, the brain uses electricity constantly to rapidly convey information via action potentials sent along axons, a biological example of electrical conductors. [2]
In the 1940s, electroconvulsive therapy (ECT), i.e. electrical brain stimulation over one or both hemispheres to create a seizure, was used in treating severe depression. [3]
In 1985, researchers stimulated - with a pulsed magnetic field - discrete regions on the surface of the brain through the skull. [4] By connecting a wire coil to a source of electric current and placing the coil on the scalp over the motor cortex, Barker et al. [4] provided the first application of transcranial magnetic stimulation (TMS). A few years later, development of stimulators able to deliver long trains of closely spaced pulses enabled repetitive transcranial magnetic stimulation (rTMS). This expansion of TMS scope from a neurophysiological probe to a tool with the potential for altering brain function increased its popularity. [5]
Growing interest in TMS and as a result, in noninvasive brain stimulation brought transcranial direct current stimulation (tDCS) into light, a technique originally applied to humans and animal models in the mid-20th century. Unlike TMS, which can produce a direct neurostimulatory effect, tDCS did not usually elicit action potentials. Instead, tDCS was thought to exhibit a modulatory effect on brain function: the externally applied electric field displaces ions within neurons, altering neuronal excitability and modulating the firing rate of individual neurons. [6]
Brain stimulation devices are now being sold directly to consumers with the promise of enhancing brain function or wellbeing; however, the short- and long-term impact of using these devices for medical and non-medical purposes is not yet clarified. [2] Most of the direct-to-consumer brain stimulation products are tDCS devices. [2]
Modalities of Stimulation[edit | edit source]
Transcranial Electrical Stimulation (TES)[edit | edit source]
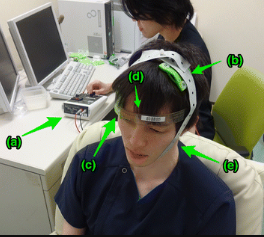
Transcranial Electrical Stimulation through tDCS devices involves the placement of electrodes pads on the head to deliver a constant low level of electric current (1-2mA), altering neural excitability. Two types of electrodes are used: anode and cathode..One of the electrodes is placed on the scalp to be over the targeted cortex region and the other is placed over the contralateral supraorbital region. [7]The direction of the produced current is from anode to cathode.
Transcranial Magnetic Stimulation (TMS)[edit | edit source]
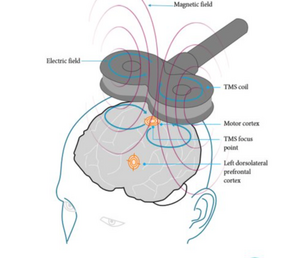
An electromagnet (circular and figure-of-8 or butterfly coil are most commonly used) is placed on the scalp to generate magnetic field pulses. When the electric current passes through this, the formed electromagnetic field passes in a perpendicular axon to the coil and produces a current, reverse and parallel to the coil.[7] The circular coil stimulates a wider brain area; the figure-of-8 coil, formed by the joining together of two circular coils, is more focused on the central mid-point and as a result, stimulation is stronger when used; a double-cone coil targets at a greater depth compared to the figure-of-8 coil and can be defined as an angulated figure-of-8 coil. [7]
In rTMS, TMS pulses are repeatedly applied to induce neuromodulation that is used to increase or decrease cortical excitability. [7] Stimulations are applied at the same dose at a specific frequency in regular sequences over the target cortical region. [8] Different parameters of intensity, duration, frequency and pattern of the stimulus may lead to changes in cortical excitability. One pulse per sec, i.e. 1 Hz, is defined as “low-frequency rTMS”; continuous administration of low frequency rTMS reduces cortical excitability. “High-frequency rTMS”, usually at a frequency of ≥5 Hz and given as bursts (sudden sequences), increases cortical excitability. [3]
The mechanism by which TMS influences brain function is not completely understood, but we do know that TMS can activate axons and cause them to fire action potentials. TMS effects are not specific to inhibitory vs. excitatory neural activity, but may change the balance between excitation and inhibition. [9][10][11]
Transcranial Ultrasound Brain Stimulation[edit | edit source]
Transcranial Ultrasound Stimulation (TUS) is an emerging noninvasive brain stimulation technique. In particular, transcranial focused ultrasound (tFUS) has attracted wide attention in neuroscience as an effective noninvasive approach to modulate brain circuits. [12]
Differences between different modes of stimulation[edit | edit source]
The main differences between different modes of stimulation are described below.
| Modality | Curent | Electric Field | Exposure to
tissue impedance |
Sensation | Effect |
|---|---|---|---|---|---|
| tDCS | Radial | Weak | Yes | Painful | Deep |
| rTMS | Parallel | Strong | No | Not painful | Superficial |
| FU | None | None | No | Not painful | Very deep |
Diagnostic use of NIBS[edit | edit source]
Outputs (i.e. motor evoked potential, central transmission time, cortical silent period, intracortical inhibition, intracortical facilitation) can also be measured to observe disease-related changes in brain activation, inhibition, or connectivity, making it a useful diagnostic tool for clinicians.[7]
References[edit | edit source]
- ↑ Fried PJ, Santarnecchi E, Antal A, Bartres-Faz D, Bestmann S, Carpenter LL, Celnik P, Edwards D, Farzan F, Fecteau S, George MS, He B, Kim YH, Leocani L, Lisanby SH, Loo C, Luber B, Nitsche MA, Paulus W, Rossi S, Rossini PM, Rothwell J, Sack AT, Thut G, Ugawa Y, Ziemann U, Hallett M, Pascual-Leone A. Training in the practice of noninvasive brain stimulation: Recommendations from an IFCN committee. Clin Neurophysiol. 2021 Mar;132(3):819-837.
- ↑ 2.0 2.1 2.2 2.3 2.4 National Institute of Neurological Disorders and Stroke. Noninvasive Brain Stimulation: Applications and Implications. 2015. Available from: https://www.ninds.nih.gov/news-events/directors-messages/all-directors-messages/noninvasive-brain-stimulation-applications-and-implications [accessed 6/12/2023]
- ↑ 3.0 3.1 Kellner CH, Greenberg RM, Murrough JW, Bryson EO, Briggs MC, Pasculli RM. ECT in treatment-resistant depression. Am J Psychiatry. 2012 Dec;169(12):1238-44.
- ↑ 4.0 4.1 Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985 May 11;1(8437):1106-7.
- ↑ Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012 Jan;133(1):98-107.
- ↑ Ukueberuwa D, Wassermann EM. Direct current brain polarization: a simple, noninvasive technique for human neuromodulation. Neuromodulation. 2010 Jul;13(3):168-73.
- ↑ 7.0 7.1 7.2 7.3 7.4 Kesikburun S. Non-invasive brain stimulation in rehabilitation. Turk J Phys Med Rehabil. 2022 Mar 1;68(1):1-8.
- ↑ Freitas C, Farzan F, Pascual-Leone A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: why, how, and what is the ultimate goal? Front. Neurosci. 2013 April ;7.
- ↑ Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil. 2009 Mar 2;6:7.
- ↑ Perini F, Cattaneo L, Carrasco M, Schwarzbach JV. Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J Neurosci. 2012 Sep 5;32(36):12361-5.
- ↑ Dayan E, Censor N, Buch ER, Sandrini M, Cohen LG. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013 Jul;16(7):838-44.
- ↑ Kim E, Anguluan E, Youn S, Kim J, Hwang J, Kim JG. Non-invasive measurement of hemodynamic change during 8 MHz transcranial focused ultrasound stimulation using near-infrared spectroscopy. BMC Neuroscience. 2019; 20: 12.