Au-Kline Syndrome: Difference between revisions
Reem Ramadan (talk | contribs) mNo edit summary |
Reem Ramadan (talk | contribs) mNo edit summary |
||
| Line 2: | Line 2: | ||
== Introduction == | == Introduction == | ||
Au–Kline syndrome (AKS) | Au–Kline syndrome (AKS) which is synonymous with Okamoto syndrome, is a multiple congenital malformation syndrome mainly associated with intellectual disability. It can be categorized as a very rare autosomal dominant genetic condition. | ||
Okamoto syndrome was first described in 1997 by Nobuhiko Okamoto et al. in Japan after observing similar symptoms and physical features in two unrelated Japanese infants. | Okamoto syndrome was first described in 1997 by Nobuhiko Okamoto et al. in Japan after observing similar symptoms and physical features in two unrelated Japanese infants<ref>Wallerstein R, Rhoads F. Natural history of a child with Okamoto syndrome. Clinical Dysmorphology. 2013 Jul 1;22(3):127-8.</ref>. Au–Kline syndrome was first described in 2015 by Ping-Yee Billie Au, Antonie D. Kline et al. after mutations in ''HNRNPK'' were found in two individuals with similar symptoms at their respective practices in Calgary, Alberta, Canada, and Baltimore, Maryland amd the United States. Both submitted the gene as a candidate to the online service GeneMatcher, which matched them together and allowed them to confirm the syndrome<ref name=":0" />. | ||
Au–Kline syndrome was first described in 2015 by Ping-Yee Billie Au, Antonie D. Kline et al. after mutations in ''HNRNPK'' were found in two individuals with similar symptoms at their respective practices in Calgary, Alberta, Canada, and Baltimore, Maryland | |||
== Clinically Relevant Anatomy == | == Clinically Relevant Anatomy == | ||
Genes are responsible for encoding proteins that in return dictate cell function and coordinate our biological functions. One of these proteins is called the Heterogeneous Nuclear Ribonucleoprotein K (HnRNP K) which is encoded by the Heterogeneous Nuclear Ribonucleoprotein K (HNRNPK), binds DNA or RNA and helps control the activity of genes and the production of proteins, helps coordinate transcriptional responses to DNA damage and plays an important role in the normal development and function of several body systems, including the [[Brain Anatomy|brain]]<ref>Mikula M, Ostrowski J. HNRNP K (heterogeneous nuclear ribonucleoprotein K). Atlas Genet Cytogenet Oncol Haematol. 2010;14(2):127-9.</ref>. | |||
== Etiology == | == Etiology == | ||
AKS is caused by mutations in the ''HNRNPK'' gene located on chromosome 9 at position q21.32, which | AKS is caused by mutations in the ''HNRNPK'' gene located on chromosome 9 at position q21.32, which leads to little or no production of HnRNP K protein. This altered gene activity and protein production disrupt the normal development and functioning of several body systems including the brain leading to intellectual disability, delayed development, and other neurological problems in people with the condition. | ||
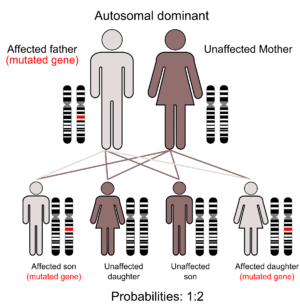
[[File:Autosomal Dominant Gene Structure.png|thumb]] | [[File:Autosomal Dominant Gene Structure.png|thumb]] | ||
It is genetically inherited in an autosomal dominant pattern. Most cases of this condition result from new (de novo) mutations in the gene that occurs during the formation of reproductive cells (eggs or sperm) in an affected individual’s parent or in early embryonic development. These cases occur in people with no history of the disorder in their family. | It is genetically inherited in an autosomal dominant pattern. Most cases of this condition result from new (de novo) mutations in the gene that occurs during the formation of reproductive cells (eggs or sperm) in an affected individual’s parent or in early embryonic development. These cases can occur in people with no history of the disorder in their family<ref>Au PYB, You J, Caluseriu O, Schwartzentruber J, Majewski J, Bernier FP, et al. GeneMatcher aids in the identification of a new malformation syndrome with intellectual disability, unique facial dysmorphisms, and skeletal and connective tissue abnormalities caused by de novo variants in HNRNPK. Hum Mutat. 2015;36:1009–14. doi: 10.1002/humu.22837.</ref>. | ||
== Clinical Presentation == | == Clinical Presentation == | ||
| Line 29: | Line 24: | ||
# Urinary tract infections, | # Urinary tract infections, | ||
# Language and walking | # Language and walking | ||
# Reduced growth: low weight and size.<ref>Au, P; Innes, M; Kline,A.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Au-Kline Syndrome", ''GeneReviews®'', University of Washington, Seattle, 2019, <nowiki>PMID 30998304</nowiki></ref> | # Reduced growth: low weight and size.<ref name=":0">Au, P; Innes, M; Kline,A.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Au-Kline Syndrome", ''GeneReviews®'', University of Washington, Seattle, 2019, <nowiki>PMID 30998304</nowiki></ref> | ||
== Diagnostic Procedures == | == Diagnostic Procedures == | ||
| Line 39: | Line 34: | ||
Sanger sequencing can confirm the nature of the mutation <ref name=":1">Au, P. Y., Goedhart, C; Ferguson, M; Breckpot, J; Devriendt, K; Wierenga, K; et al. "Phenotypic spectrum of Au-Kline syndrome: a report of six new cases and review of the literature". ''European Journal of Human Genetics''. Sept. 2018. 26 (9): 1272–1281. doi:10.1038/s41431-018-0187-2. ISSN 1476-5438. PMC 6117294. <nowiki>PMID 29904177</nowiki></ref>.<br> | Sanger sequencing can confirm the nature of the mutation <ref name=":1">Au, P. Y., Goedhart, C; Ferguson, M; Breckpot, J; Devriendt, K; Wierenga, K; et al. "Phenotypic spectrum of Au-Kline syndrome: a report of six new cases and review of the literature". ''European Journal of Human Genetics''. Sept. 2018. 26 (9): 1272–1281. doi:10.1038/s41431-018-0187-2. ISSN 1476-5438. PMC 6117294. <nowiki>PMID 29904177</nowiki></ref>.<br> | ||
== Management | == Management == | ||
The management depends on the symptoms. Physiotherapy management may be suggested for low tone and functional growth. The rehabilitation team should include a team of cardiologists, neurologists, physiotherapists, occupational therapists, special educators, speech therapists, and other professionals associated with the symptoms. | The management depends on the symptoms of each patient. Physiotherapy management may be suggested for low tone and functional growth. The rehabilitation team should include a team of cardiologists, neurologists, physiotherapists, urologists, occupational therapists, special educators, speech therapists, and other professionals associated with the symptoms.[[Genetics and Health|Genetic counselling]] is an important aspect of management for this condition. Prenatal testing for pregnancies at increased risk is possible if the HNRNPK pathogenic variant in the family is known. The prognosis of the condition is yet vague due to a handful of diagnosed individuals<ref name=":1" />. | ||
[[Genetics and Health|Genetic counselling]] is an important aspect of management for this condition. Prenatal testing for pregnancies at increased risk is possible if the HNRNPK pathogenic variant in the family is known. | |||
The prognosis of the condition is yet vague due to a handful of diagnosed individuals<ref name=":1" />. | |||
== References == | == References == | ||
Revision as of 13:44, 16 August 2023
Top Contributors - Rucha Gadgil, Reem Ramadan and Uchechukwu Chukwuemeka
Introduction[edit | edit source]
Au–Kline syndrome (AKS) which is synonymous with Okamoto syndrome, is a multiple congenital malformation syndrome mainly associated with intellectual disability. It can be categorized as a very rare autosomal dominant genetic condition.
Okamoto syndrome was first described in 1997 by Nobuhiko Okamoto et al. in Japan after observing similar symptoms and physical features in two unrelated Japanese infants[1]. Au–Kline syndrome was first described in 2015 by Ping-Yee Billie Au, Antonie D. Kline et al. after mutations in HNRNPK were found in two individuals with similar symptoms at their respective practices in Calgary, Alberta, Canada, and Baltimore, Maryland amd the United States. Both submitted the gene as a candidate to the online service GeneMatcher, which matched them together and allowed them to confirm the syndrome[2].
Clinically Relevant Anatomy[edit | edit source]
Genes are responsible for encoding proteins that in return dictate cell function and coordinate our biological functions. One of these proteins is called the Heterogeneous Nuclear Ribonucleoprotein K (HnRNP K) which is encoded by the Heterogeneous Nuclear Ribonucleoprotein K (HNRNPK), binds DNA or RNA and helps control the activity of genes and the production of proteins, helps coordinate transcriptional responses to DNA damage and plays an important role in the normal development and function of several body systems, including the brain[3].
Etiology[edit | edit source]
AKS is caused by mutations in the HNRNPK gene located on chromosome 9 at position q21.32, which leads to little or no production of HnRNP K protein. This altered gene activity and protein production disrupt the normal development and functioning of several body systems including the brain leading to intellectual disability, delayed development, and other neurological problems in people with the condition.
It is genetically inherited in an autosomal dominant pattern. Most cases of this condition result from new (de novo) mutations in the gene that occurs during the formation of reproductive cells (eggs or sperm) in an affected individual’s parent or in early embryonic development. These cases can occur in people with no history of the disorder in their family[4].
Clinical Presentation[edit | edit source]
This condition has been characterized by:
- Congenital hydronephrosis,
- Low muscle tone and reduced reflexes,
- Heart defects: like aortic valve stenosis, atrial or ventricular septal defect, bicuspid aortic valve or patent ductus arteriosus.
- Intellectual disability,
- Characteristic facial features: prominent, downturned ears, an open, downturned mouth, and drooping eyelids (ptosis)
- Neurological and skeletal abnormalities,
- Urinary tract infections,
- Language and walking
- Reduced growth: low weight and size.[2]
Diagnostic Procedures[edit | edit source]
The condition can be diagnosed by physical symptoms and confirmed by genetic testing. Genetic testing can be done by:
- whole exome sequencing, and
- comparative genomic hybridization (for microdeletions).
Sanger sequencing can confirm the nature of the mutation [5].
Management[edit | edit source]
The management depends on the symptoms of each patient. Physiotherapy management may be suggested for low tone and functional growth. The rehabilitation team should include a team of cardiologists, neurologists, physiotherapists, urologists, occupational therapists, special educators, speech therapists, and other professionals associated with the symptoms.Genetic counselling is an important aspect of management for this condition. Prenatal testing for pregnancies at increased risk is possible if the HNRNPK pathogenic variant in the family is known. The prognosis of the condition is yet vague due to a handful of diagnosed individuals[5].
References[edit | edit source]
- ↑ Wallerstein R, Rhoads F. Natural history of a child with Okamoto syndrome. Clinical Dysmorphology. 2013 Jul 1;22(3):127-8.
- ↑ 2.0 2.1 Au, P; Innes, M; Kline,A.; Ardinger, Holly H.; Pagon, Roberta A.; Wallace, Stephanie E. (eds.), "Au-Kline Syndrome", GeneReviews®, University of Washington, Seattle, 2019, PMID 30998304
- ↑ Mikula M, Ostrowski J. HNRNP K (heterogeneous nuclear ribonucleoprotein K). Atlas Genet Cytogenet Oncol Haematol. 2010;14(2):127-9.
- ↑ Au PYB, You J, Caluseriu O, Schwartzentruber J, Majewski J, Bernier FP, et al. GeneMatcher aids in the identification of a new malformation syndrome with intellectual disability, unique facial dysmorphisms, and skeletal and connective tissue abnormalities caused by de novo variants in HNRNPK. Hum Mutat. 2015;36:1009–14. doi: 10.1002/humu.22837.
- ↑ 5.0 5.1 Au, P. Y., Goedhart, C; Ferguson, M; Breckpot, J; Devriendt, K; Wierenga, K; et al. "Phenotypic spectrum of Au-Kline syndrome: a report of six new cases and review of the literature". European Journal of Human Genetics. Sept. 2018. 26 (9): 1272–1281. doi:10.1038/s41431-018-0187-2. ISSN 1476-5438. PMC 6117294. PMID 29904177