Wilson's Disease: A Case Study: Difference between revisions
Courtney Lim (talk | contribs) No edit summary |
Naiya Patel (talk | contribs) No edit summary |
||
| Line 2: | Line 2: | ||
'''Original Editor ''' -[[Yuka Abe]] | '''Original Editor ''' -[[Yuka Abe]] | ||
'''Top Contributors''' - Courtney Lim, Jocelyn Chung | '''Top Contributors''' - Courtney Lim, Jocelyn Chung, Naiya Patel | ||
</div> | </div> | ||
| Line 30: | Line 30: | ||
== Intervention == | == Intervention == | ||
Although we cannot cure Wilson’s disease, we can treat the underlying neurological and motor impairments that affect a person’s activities of daily living and their overall quality of life. Through physical therapy interventions, we can treat symptoms such as decreased range of motion, rigidity, tremors, dystonia, poor balance, and abnormal gait patterns as well as educate the patient on adaptive and energy conservation techniques to improve endurance and activity tolerance. Currently, Wilson’s disease is quite rare and requires further research in rehabilitation therapies. However, because Wilson’s disease has similar symptoms to other neuromotor conditions such as Parkinson’s disease and Stroke, we have implemented transferrable research findings to support our therapeutic interventions. | |||
Frequency: Outpatient rehab twice per week, and home exercise plan to be completed 3 times per week. | |||
A case report by Maiarú et al. (2016) focused on physical therapy in a 32-year-old male who was diagnosed with Wilson’s Disease approximately 16 years ago from the time of the study. In this case report the authors describe the use of exercises to improve static and dynamic balance as well as functional capacity. These therapies are transferrable to our patient case with certain modifications. To address our patient’s goal of improving balance and gait, we can implement both static and dynamic balance exercises. Maiarú et al. (2016) implemented static standing balance exercises on a hard surface starting with feet together, and progressing to semi-tandem, tandem, and single-leg stance. To further progress and challenge our patient, we can manipulate somatosensory aspects such as eyes open vs closed, making the surface unsteady (foam), or use or perturbations (internal/external). Standing exercises can each be performed for 1-minute durations, in 4 series, with breaks in between or as needed (Maiarú et al., 2016). | |||
In terms of motor presentation, Wilson’s disease has some overlapping presentations as Parkinson’s disease, a condition in which there is substantial research on physical therapy. For dynamic balance and gait training, there is research on Parkinson’s disease that indicates treadmill walking as well as dance therapy shows improvements in gait as well as improved scores on Berg Balance scale (Boonstra & Bloem, 2008). Therefore, to improve our patient’s dynamic balance and gait, we can implement combined sessions of treadmill walking on low-medium speeds for 25–30-minutes and dance sessions for 25–30-minutes, such as tango or large movement dances. These activities can be performed in bouts of 3 minutes with 5-minute breaks or as the patient tolerates, totalling up to 1 hour per session as suggested by Maiarú et al. (2016). Ambulation in open or closed environments can also be initiated once patient has improved dynamic balance, in order to improve function at home and in the community. | |||
A common finding with Wilson’s disease is tremors of the hands which can be very disruptive to daily living and participation in activities. For our patient’s goal of being able to golf we can implement strengthening and range of motion exercises for the upper extremities, with a focus on eccentric exercises for controlling tremors of the hand. A randomized control trial by Kadkhodaie et al., (2020) found that progressive eccentric exercises of the upper extremity can improve resting hand tremors as seen in Parkinson’s disease. Based on this research, we are inferring that this intervention could possibly have similar effects on tremors seen in Wilson’s disease. To add on, the use of a Tremor glove has been supported by research, specifically, a systemic review by Shahien et al., (2023), found that tremor glove stimulation can reduce the angular velocity and peak magnitude of tremors in individuals with Parkinson’s disease. In addition, as golfing requires walking and carrying a golf club or picking up/placing down a golf ball, we can incorporate a combination of motor and cognitive dual task training which studies have found is effective in improving balance and gait in chronic stroke patients than when performed separately (An et al., 2014) | |||
To improve flexion of the lower extremities, specifically at the ankle, we can implement seated range of motion exercises, focusing on dorsiflexion of the ankles and progressing the exercises by using different TheraBands (increasing resistance). Seated lower extremity exercises can be performed daily for 1-2 sets of 8-10 reps or as tolerated, and can include, ankle plantar flexion/ dorsiflexion, knee extension/ flexion, hip flexion/abduction. To add on, reduced ankle dorsiflexion can be treated using Mulligan’s mobilization with movement technique (MWM). A systemic review of RCTs was conducted by Alamer et al., (2021), that found MWM showed improvements in range of motion, balance, and gait functions among individuals with chronic stroke. Parameters based on this systemic review could generally be grade III mobilization with 3 sets of 10 repetitions of ankle dorsiflexion, 3 times per week (Alamer et al., 2021). | |||
To treat more functional aspects of the patient’s life such as lifting a glass of water or independent feeding, we can implement functional capacity training as indicated in the case report mentioned above by Maiarú et al. (2016). Functional capacity training for our patient can include standing at counter and stacking different sized cups, placing cups on shelf, and picking up cups with water or with weights to imitate the presence of liquids in a cup. This task can be progressed by changing cup sizes and the heaviness of the cup, as well as the height of the counter or shelves to which the patient would reach for the cup. In addition, this task can be performed with minimal hand on hand assistance (active assisted) depending on the extent of the hand tremor, to help initiate and control the desired movement and then progress to independent (active) movement. To add on, the task of feeding can be practiced by sitting at a table and picking up a utensil then repeating the movement pattern of bringing a utensil to the mouth and back to the table. This type of intervention can be dosed based on patient tolerance and be performed in bouts of 3-5 minutes with rests in between, totaling 30 minutes and ending with 15 minutes of stretching the hand/ upper extremity (Maiarú et al., 2016). | |||
There is insufficient research on physiotherapy treatment of orofacial dyskinesia in Wilson’s disease, however there is research on facial rehabilitation strategies in patients with facial palsy. Although the conditions are not equivalent, the goal of re-education and control of facial muscles is the same, therefore we could attempt to use similar interventions. As the patient has a goal to voluntarily smile and chew food, our focus is on targeting movement and strength of the orofacial muscles. Research conducted by Robinson & Baiungo (2018) suggests several interventions including the use of soft tissue mobilization to target muscle tightness, neuromuscular retraining/ re-education to help facilitate the return of normal facial movement patterns while decreasing unwanted movements, and emphasizing self-management strategies so patients can identify, develop, and refine proper facial movement patterns and expressions. According to Hegde et al., (2014), orofacial physiotherapy can include soft tissue mobilization, joint mobilization, and muscle conditioning. Focusing on strengthening, our patient can perform the following facial exercises daily: puckering the lips, smiling with teeth and gums showing, puffing the cheeks with air, pursed lips, lifting lower lip by pouting, holding a straw or stick between the lips (Hegde et al., 2014). For the tongue, exercises include sticking the tongue out straight, to the left and right, and up and down, sweeping the tongue along the teeth and gums, and pushing the tongue against the cheeks (Hegde et al., 2014). | |||
Although intervention plans target current problems and limitations of the patient, we must also consider the possibility of adverse effects and ways in which we can modify our plans to best support our patient. A report by Caniça et al., (2022) indicates that falls are often the number one adverse event reported either during, or after physiotherapy interventions among Parkinson’s patients. As our patient presents with parkinsonism signs, they could also be at a higher risk of falls. If our patient were to experience a fall as an adverse event, the time and intensity of the physiotherapy intervention plan could be reduced. For example, we would still want the frequency to be maintained however we can adjust how long we are performing exercises or mobility and how much resistance is applied. The intervention plan can be modified to be performed in sitting until the patient feels comfortable performing exercises in standing or standing can be performed in shorter bouts with increased rest periods. To add on, we can incorporate additional rest breaks and build up the patient’s tolerance if they present with a lower activity tolerance after their fall. In addition, if the patient has weakness or soreness, we can offer modalities such as heat, ice, or rest that can help with any swelling or bruising which may comfort them and keep them motivated to continue therapy. An important factor to consider is also how the patient fell or what the source or trigger was and then to incorporate a plan to prevent the fall from reoccurring in the future. Increasing safety is an effective step as it is our responsibility as physiotherapists to ensure the patient’s safety as well and comfort and tolerance with the interventions. | |||
Virtual reality (VR) is a new and upcoming innovative technology that has been shown to help facilitate and improve the rehabilitation process. Virtual reality uses principles of neuroplasticity and motor learning and utilizes computer algorithms to make decisions towards progressing the difficulty of a task<ref name=":2">Weiss, P. (Tamar) L., Keshner, E. A., & Levin, M. F. (2014). Virtual Reality for Physical and Motor Rehabilitation. Springer New York, 222-225.</ref>. It has the ability to track neuroplastic changes and can be used to track recovery<ref name=":2" />. Studies have shown VR to have great ecological validity and researchers have been steadily improving its technology (e.g higher resolution 3D systems are more prevalent)<ref name=":2" />. For instance, new research has worked to improve head mounted displays, allowing these displays to produce realistic, high resolution augmented visual feedback<ref name=":2" />. This visual feedback can help with sensorimotor integration and thus help the patient improve their gait<ref name=":2" />. Our patient, Chris Brown, presents with gait impairments. VR technology can be used to help in the treatment process of our patient’s gait patterning. Another applicable example is that VR can be used to improve upper extremity functioning and fine motor control by providing hepatic feedback to the patient<ref name=":2" />. This can also help to facilitate Chris Brown's rehabilitation process. VR is a fresh new rehabilitation approach that can be engaging for patients and could help with motivation. | Virtual reality (VR) is a new and upcoming innovative technology that has been shown to help facilitate and improve the rehabilitation process. Virtual reality uses principles of neuroplasticity and motor learning and utilizes computer algorithms to make decisions towards progressing the difficulty of a task<ref name=":2">Weiss, P. (Tamar) L., Keshner, E. A., & Levin, M. F. (2014). Virtual Reality for Physical and Motor Rehabilitation. Springer New York, 222-225.</ref>. It has the ability to track neuroplastic changes and can be used to track recovery<ref name=":2" />. Studies have shown VR to have great ecological validity and researchers have been steadily improving its technology (e.g higher resolution 3D systems are more prevalent)<ref name=":2" />. For instance, new research has worked to improve head mounted displays, allowing these displays to produce realistic, high resolution augmented visual feedback<ref name=":2" />. This visual feedback can help with sensorimotor integration and thus help the patient improve their gait<ref name=":2" />. Our patient, Chris Brown, presents with gait impairments. VR technology can be used to help in the treatment process of our patient’s gait patterning. Another applicable example is that VR can be used to improve upper extremity functioning and fine motor control by providing hepatic feedback to the patient<ref name=":2" />. This can also help to facilitate Chris Brown's rehabilitation process. VR is a fresh new rehabilitation approach that can be engaging for patients and could help with motivation. | ||
Revision as of 17:12, 11 May 2023
Original Editor -Yuka Abe
Top Contributors - Courtney Lim, Jocelyn Chung, Naiya Patel
Abstract[edit | edit source]
Introduction[edit | edit source]
Wilson’s Disease is a genetic disorder involving an excess of copper in the body, due to the genetic mutation of the ATP7B gene (13,17). This rare neurological condition commonly affects those between the age of 10-20 years, however those younger than 5 years and older than 70 years are able to be diagnosed as well (14,16). It predominantly affects the liver and brain, where neurological and hepatic symptoms are said to be equivalent in prevalence among patients, however symptoms differ in severity and characteristics depending on which organ is involved (15). The range of symptoms makes diagnoses hard for patients with Wilson’s Disease, as symptoms can be solely hepatic, neurological or psychological, or a combination of them (12). Common neurologic symptoms of patients with Wilson’s Disease are dysarthria, ataxia, dystonia, parkinsonism, chorea, and tremors (12).
The purpose of this case study is to inform readers about the presentation of Wilson’s Disease in a previously active and young individual recently diagnosed with this condition. This study also dissects the role of physiotherapy and its effect on moderating symptoms and improving function along with quality of life in the individual with Wilson’s Disease. Furthermore, this case study provides the understanding of the significant role of non-physiotherapist members and interdisciplinary teams in the care of individuals with neurological conditions such as Wilson’s disease. Lastly, this case study bestows the online translation of knowledge within the scope of physiotherapy, and aids in the understanding of technology in neurological rehabilitation.
Client Characteristics[edit | edit source]
The patient is a 28-year-old male, who is an accountant who lives independently in Toronto, Ontario. His hobbies include golfing on the weekend with friends. The patient was diagnosed with Wilson's Disease 1 month ago and was referred to physiotherapy by a neurologist. He has been receiving physiotherapy treatment for 2 weeks. His main complaints are limitations on standing tolerance at work and on sports participation. He has noticed changes in his balance and sensation in his feet.
Examination Findings[edit | edit source]
Outcome Measures[edit | edit source]
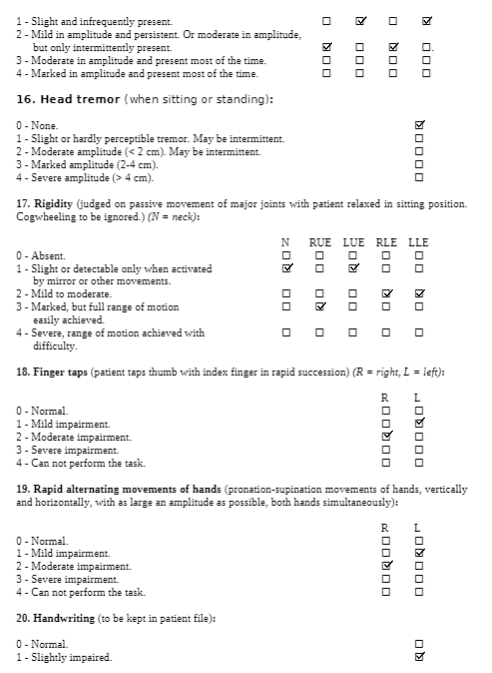
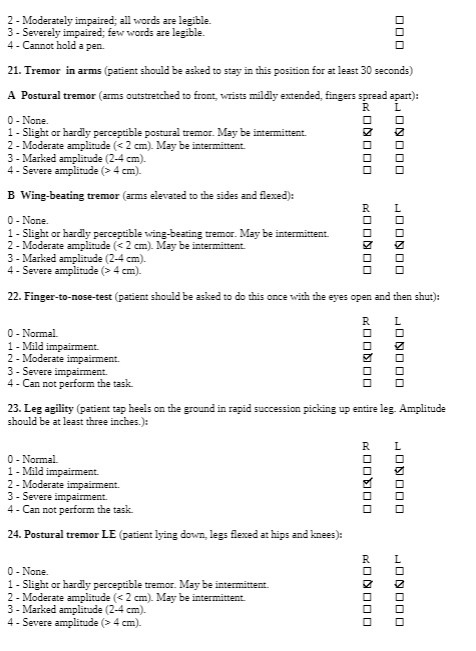
The Unified Wilson’s Disease Rating Scale (UWDRS) would be appropriate for this patient in measuring the body structure and function limitations. The UWDRS consists of 3 subscales – neurological, hepatic, and psychiatric. The neurological subscale consists of items including rigidity, chorea, tremors, as well as facial expression and oromandibular dystonia. The UWDRS has been shown to be a reliable and valid tool to monitor disease progression in patients with Wilson’s disease [1]. Items on the UWDRS were adapted from scales that have already been established in measuring neurological impairments, including the Unified Parkinson’s Disease Rating Scale (UPDRS), Barthel index, and the Unified Huntington’s Disease Rating Scale, which suggests a high degree of content validity[1].
To evaluate the activity limitation, the 2 Minute Walk Test (2MWT) can be used. The 2MWT measures the distance walked in 2 minutes, and can be used to quantify walking limitations and fatigue. This measure has been proven to be reliable and valid in patients with various neuromuscular diseases[2]. In addition, the 2MWT is well correlated with the 6 Minute Walk Test, which is a widely used and validated tool in measuring walking capability. For this case, the patient is currently unable to walk without assistance, so the 2MWT would be administered when the patient is able to walk independently.
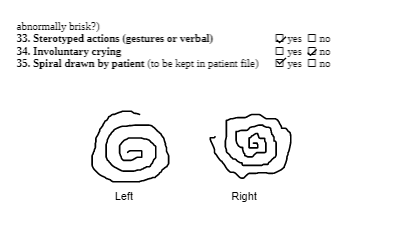
For participation limitations, the Tremor Research Group Essential Tremor Rating Scale (TETRAS) can be used to measure essential tremors. The TETRAS consists of ADL (12 items) and performance (9 items) sections[3]. Each item is scored on a scale of 0 to 4, where a higher score indicates more severe tremors. It is a well-validated and reliable tool to assess the severity of essential tremors, which is commonly present in those with Wilson’s Disease[4][5] . Furthermore, the TETRAS assesses wing-beating tremors which is characteristic of Wilson’s Disease.
Common Comorbidity to Screen For[edit | edit source]
Psychiatric disorders, specifically depression, is a common comorbidity that should be screened in patients with Wilson’s Disease (WD). Evidence shows that there is a higher prevalence of depression in those with WD as compared to those with other chronic diseases that live with a similar level of disability[6]. Psychiatric disorders as a comorbidity have a poorer prognosis as these complicate the course of WD with significant impact on quality of life[7]. Therefore, it is necessary to screen depression in patients with WD. Physiotherapists (PT) can use many assessments, such as Beck’s Depression Inventory, a self-reported scale, to which the PT can refer on if necessary.
Clinical Hypothesis[edit | edit source]
Intervention[edit | edit source]
Although we cannot cure Wilson’s disease, we can treat the underlying neurological and motor impairments that affect a person’s activities of daily living and their overall quality of life. Through physical therapy interventions, we can treat symptoms such as decreased range of motion, rigidity, tremors, dystonia, poor balance, and abnormal gait patterns as well as educate the patient on adaptive and energy conservation techniques to improve endurance and activity tolerance. Currently, Wilson’s disease is quite rare and requires further research in rehabilitation therapies. However, because Wilson’s disease has similar symptoms to other neuromotor conditions such as Parkinson’s disease and Stroke, we have implemented transferrable research findings to support our therapeutic interventions.
Frequency: Outpatient rehab twice per week, and home exercise plan to be completed 3 times per week.
A case report by Maiarú et al. (2016) focused on physical therapy in a 32-year-old male who was diagnosed with Wilson’s Disease approximately 16 years ago from the time of the study. In this case report the authors describe the use of exercises to improve static and dynamic balance as well as functional capacity. These therapies are transferrable to our patient case with certain modifications. To address our patient’s goal of improving balance and gait, we can implement both static and dynamic balance exercises. Maiarú et al. (2016) implemented static standing balance exercises on a hard surface starting with feet together, and progressing to semi-tandem, tandem, and single-leg stance. To further progress and challenge our patient, we can manipulate somatosensory aspects such as eyes open vs closed, making the surface unsteady (foam), or use or perturbations (internal/external). Standing exercises can each be performed for 1-minute durations, in 4 series, with breaks in between or as needed (Maiarú et al., 2016).
In terms of motor presentation, Wilson’s disease has some overlapping presentations as Parkinson’s disease, a condition in which there is substantial research on physical therapy. For dynamic balance and gait training, there is research on Parkinson’s disease that indicates treadmill walking as well as dance therapy shows improvements in gait as well as improved scores on Berg Balance scale (Boonstra & Bloem, 2008). Therefore, to improve our patient’s dynamic balance and gait, we can implement combined sessions of treadmill walking on low-medium speeds for 25–30-minutes and dance sessions for 25–30-minutes, such as tango or large movement dances. These activities can be performed in bouts of 3 minutes with 5-minute breaks or as the patient tolerates, totalling up to 1 hour per session as suggested by Maiarú et al. (2016). Ambulation in open or closed environments can also be initiated once patient has improved dynamic balance, in order to improve function at home and in the community.
A common finding with Wilson’s disease is tremors of the hands which can be very disruptive to daily living and participation in activities. For our patient’s goal of being able to golf we can implement strengthening and range of motion exercises for the upper extremities, with a focus on eccentric exercises for controlling tremors of the hand. A randomized control trial by Kadkhodaie et al., (2020) found that progressive eccentric exercises of the upper extremity can improve resting hand tremors as seen in Parkinson’s disease. Based on this research, we are inferring that this intervention could possibly have similar effects on tremors seen in Wilson’s disease. To add on, the use of a Tremor glove has been supported by research, specifically, a systemic review by Shahien et al., (2023), found that tremor glove stimulation can reduce the angular velocity and peak magnitude of tremors in individuals with Parkinson’s disease. In addition, as golfing requires walking and carrying a golf club or picking up/placing down a golf ball, we can incorporate a combination of motor and cognitive dual task training which studies have found is effective in improving balance and gait in chronic stroke patients than when performed separately (An et al., 2014)
To improve flexion of the lower extremities, specifically at the ankle, we can implement seated range of motion exercises, focusing on dorsiflexion of the ankles and progressing the exercises by using different TheraBands (increasing resistance). Seated lower extremity exercises can be performed daily for 1-2 sets of 8-10 reps or as tolerated, and can include, ankle plantar flexion/ dorsiflexion, knee extension/ flexion, hip flexion/abduction. To add on, reduced ankle dorsiflexion can be treated using Mulligan’s mobilization with movement technique (MWM). A systemic review of RCTs was conducted by Alamer et al., (2021), that found MWM showed improvements in range of motion, balance, and gait functions among individuals with chronic stroke. Parameters based on this systemic review could generally be grade III mobilization with 3 sets of 10 repetitions of ankle dorsiflexion, 3 times per week (Alamer et al., 2021).
To treat more functional aspects of the patient’s life such as lifting a glass of water or independent feeding, we can implement functional capacity training as indicated in the case report mentioned above by Maiarú et al. (2016). Functional capacity training for our patient can include standing at counter and stacking different sized cups, placing cups on shelf, and picking up cups with water or with weights to imitate the presence of liquids in a cup. This task can be progressed by changing cup sizes and the heaviness of the cup, as well as the height of the counter or shelves to which the patient would reach for the cup. In addition, this task can be performed with minimal hand on hand assistance (active assisted) depending on the extent of the hand tremor, to help initiate and control the desired movement and then progress to independent (active) movement. To add on, the task of feeding can be practiced by sitting at a table and picking up a utensil then repeating the movement pattern of bringing a utensil to the mouth and back to the table. This type of intervention can be dosed based on patient tolerance and be performed in bouts of 3-5 minutes with rests in between, totaling 30 minutes and ending with 15 minutes of stretching the hand/ upper extremity (Maiarú et al., 2016).
There is insufficient research on physiotherapy treatment of orofacial dyskinesia in Wilson’s disease, however there is research on facial rehabilitation strategies in patients with facial palsy. Although the conditions are not equivalent, the goal of re-education and control of facial muscles is the same, therefore we could attempt to use similar interventions. As the patient has a goal to voluntarily smile and chew food, our focus is on targeting movement and strength of the orofacial muscles. Research conducted by Robinson & Baiungo (2018) suggests several interventions including the use of soft tissue mobilization to target muscle tightness, neuromuscular retraining/ re-education to help facilitate the return of normal facial movement patterns while decreasing unwanted movements, and emphasizing self-management strategies so patients can identify, develop, and refine proper facial movement patterns and expressions. According to Hegde et al., (2014), orofacial physiotherapy can include soft tissue mobilization, joint mobilization, and muscle conditioning. Focusing on strengthening, our patient can perform the following facial exercises daily: puckering the lips, smiling with teeth and gums showing, puffing the cheeks with air, pursed lips, lifting lower lip by pouting, holding a straw or stick between the lips (Hegde et al., 2014). For the tongue, exercises include sticking the tongue out straight, to the left and right, and up and down, sweeping the tongue along the teeth and gums, and pushing the tongue against the cheeks (Hegde et al., 2014).
Although intervention plans target current problems and limitations of the patient, we must also consider the possibility of adverse effects and ways in which we can modify our plans to best support our patient. A report by Caniça et al., (2022) indicates that falls are often the number one adverse event reported either during, or after physiotherapy interventions among Parkinson’s patients. As our patient presents with parkinsonism signs, they could also be at a higher risk of falls. If our patient were to experience a fall as an adverse event, the time and intensity of the physiotherapy intervention plan could be reduced. For example, we would still want the frequency to be maintained however we can adjust how long we are performing exercises or mobility and how much resistance is applied. The intervention plan can be modified to be performed in sitting until the patient feels comfortable performing exercises in standing or standing can be performed in shorter bouts with increased rest periods. To add on, we can incorporate additional rest breaks and build up the patient’s tolerance if they present with a lower activity tolerance after their fall. In addition, if the patient has weakness or soreness, we can offer modalities such as heat, ice, or rest that can help with any swelling or bruising which may comfort them and keep them motivated to continue therapy. An important factor to consider is also how the patient fell or what the source or trigger was and then to incorporate a plan to prevent the fall from reoccurring in the future. Increasing safety is an effective step as it is our responsibility as physiotherapists to ensure the patient’s safety as well and comfort and tolerance with the interventions.
Virtual reality (VR) is a new and upcoming innovative technology that has been shown to help facilitate and improve the rehabilitation process. Virtual reality uses principles of neuroplasticity and motor learning and utilizes computer algorithms to make decisions towards progressing the difficulty of a task[8]. It has the ability to track neuroplastic changes and can be used to track recovery[8]. Studies have shown VR to have great ecological validity and researchers have been steadily improving its technology (e.g higher resolution 3D systems are more prevalent)[8]. For instance, new research has worked to improve head mounted displays, allowing these displays to produce realistic, high resolution augmented visual feedback[8]. This visual feedback can help with sensorimotor integration and thus help the patient improve their gait[8]. Our patient, Chris Brown, presents with gait impairments. VR technology can be used to help in the treatment process of our patient’s gait patterning. Another applicable example is that VR can be used to improve upper extremity functioning and fine motor control by providing hepatic feedback to the patient[8]. This can also help to facilitate Chris Brown's rehabilitation process. VR is a fresh new rehabilitation approach that can be engaging for patients and could help with motivation.
Despite the benefits that VR can bring, it does come with a few challenges. Two of the most pressing issues include accessibility issues due to expensive equipment costs and safety issues and considerations[9].
To mitigate costs barriers associated with VR systems, facilities/clinicians can work with researchers in pilot programs[9]. This is a great way for the clinician and facilities to see if VR is suitable for their patient population while assessing if VR would be a reasonable investment that ultimately will save money in the long-term[9]. Cost barriers can also be addressed by using alternative materials such as a cardboard headset[9]. Some VR training content could also be used in a more accessible form such as on a desktop or mobile device[9].
To mitigate issues with safety, proper clinician training and education would be appropriate. To use VR, there could be requirements such as completion of a training course or passing a specific exam[9]. The patient also needs to be given proper comprehensive education and informed consent. Side effects of VR can include cybersickness, perceptuomotor after-effects, headaches and eye-strain[9]. The patient is also at risk for addiction to VR-based technologies[9]. The patient should be informed of both benefits and drawbacks of the treatment, as well as alternative solutions. This can help to ensure safety and proper usage of VR systems by both patient and practitioner.
Goals[edit | edit source]
Problem 1: Unable to achieve optimal flexion in the lower extremity, especially dorsiflexion of the ankle
- Short term goal: In 2 weeks, Chris will be able to increase dorsiflexion at the ankle to 10 degrees as measured by goniometry
- Long term goal: Chris will be able to heel strike in his gait cycle without an AFO for discharge
Problem 2: Dyskinesia, affecting his ability to use his face and mouth muscles voluntarily
- Short term goal: In 2 weeks, Chris will have voluntary control of his face muscles as shown by a smile on his face
- Long term goal: Chris will have voluntary control of his mouth muscles as he will be able to chew solid foods for two meals a day for discharge
Problem 3: Hypertonia, specifically rigidity, chorea and tremors
- Short term goal: In 2 weeks, Chris will be able to pick up a glass of water with minimal tremors as measured by minimal spillage of water
- Long term goal: Chris will be able to feed himself independently for discharge
Problem 4: Unable to walk without moderate amount of assistance due to muscle fatigue and lack of balance and coordination
- Short term goal: In 2 weeks, Chris will improve standing balance as measured with improvement on the BERG balance scale
- Long term goal: Chris will be able to walk 30 meters independent with a gait aid for discharge
Problem 5: Unable to golf with his friends on the weekends due to essential tremors
- Short term goal: In 2 weeks, Chris will be able to put a golf ball 1 meter away from the putter
- Long term goal: Chris will be able to perform a golf swing with a golf club for discharge
Discharge date: projected 6 months from admission to outpatient rehabilitation – will be dependent on trajectory of patient progression.
Outcome[edit | edit source]
UWDRS Outcome:
UWDRS Score Part I: 0
UWDRS Score Part II: 23
UWDRS Score Part III:
2MWT: Unable to complete at this time as patient requires assistance when walking
TETRAS Outcome:
TETRAS Score ADLs: 30
TETRAS Score Performance: 39.5
Referrals[edit | edit source]
3 other healthcare professionals/services that can be involved in the client’s care include:
-speech language pathology
-occupational therapy
-psychiatric care and counseling
Speech language therapy would be the priority recommendation for Chris Brown as they address problems regarding communication, swallowing, and eating[10]. The most pressing issue would be swallowing and eating as these skills are crucial for survival and it is important to know the patient’s functional level to provide adequate support and mitigate risks such as aspiration or choking. The speech language therapist can assess oral muscle functioning and strength for speaking and swallowing[10]. Based off this information, they can make recommendations for an appropriate diet and can create a treatment plan to help improve independence with eating and swallowing[10]. The speech language therapist can help with verbal and non-verbal communication, recovery of speech, language, and memory skills[10]. These skills are essential for daily living and improving these skills will contribute to an overall improved quality of life and well-being. Working on these skills will be instrumental in success with other therapies (physical therapy, occupational therapy, psychiatric care and counseling).
Discussion[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 Leinweber, B., Möller, J. C., Scherag, A., Reuner, U., Günther, P., Lang, C. J. G., Schmidt, H. H. J., Schrader, C., Bandmann, O., Czlonkowska, A., Oertel, W. H., & Hefter, H. (2008). Evaluation of the Unified Wilson’s Disease Rating Scale (UWDRS) in German patients with treated Wilson’s disease. Movement Disorders, 23(1), 54–62. https://doi.org/10.1002/mds.21761
- ↑ Andersen, L. K., Knak, K. L., Witting, N., & Vissing, J. (2016). Two- and 6-minute walk tests assess walking capability equally in neuromuscular diseases. Neurology, 86(5), 442–445. https://doi.org/10.1212/WNL.0000000000002332
- ↑ Elble, R. J. (2016). The Essential Tremor Rating Assessment Scale. Journal of Neurology & Neuromedicine, 1(4). https://www.jneurology.com/articles/the-essential-tremor-rating-assessment-scale.html
- ↑ Ondo, W., Hashem, V., LeWitt, P. A., Pahwa, R., Shih, L., Tarsy, D., Zesiewicz, T., & Elble, R. (2018). Comparison of the Fahn-Tolosa-Marin Clinical Rating Scale and the Essential Tremor Rating Assessment Scale. Movement Disorders Clinical Practice, 5(1), 60–65. https://doi.org/10.1002/mdc3.12560
- ↑ Ortiz, J. F., Morillo Cox, Á., Tambo, W., Eskander, N., Wirth, M., Valdez, M., & Niño, M. (n.d.). Neurological Manifestations of Wilson’s Disease: Pathophysiology and Localization of Each Component. Cureus, 12(11), e11509. https://doi.org/10.7759/cureus.11509
- ↑ Ehmann, T.S., Beninger, R.J., Gawel, M.J., & Riopelle, R.J. (1990). Depressive symptoms in Parkinson's disease: A comparison with disabled control subjects. Journal of Geriatric Psychiatry and Neurology, 3, 3–9. doi:10.1177/ 089198879000300102
- ↑ Carta, M.G., Mura, G., Sorbello, O., Farina, G., & Demelia, L. (2012). Quality of life and psychiatric symptoms in Wilson's Disease: The Relevance of Bipolar Disorders. Clinical Practice & Epidemiology in Mental Health, 8, 102–109
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Weiss, P. (Tamar) L., Keshner, E. A., & Levin, M. F. (2014). Virtual Reality for Physical and Motor Rehabilitation. Springer New York, 222-225.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Baniasadi, T., Ayyoubzadeh, S. M., & Mohammadzadeh, N. (2020). Challenges and Practical Considerations in Applying Virtual Reality in Medical Education and Treatment. Oman medical journal, 35(3), e125. https://doi.org/10.5001/omj.2020.43
- ↑ 10.0 10.1 10.2 10.3 Other therapies. Wilson Disease Association. (2022, February 21). https://wilsondisease.org/medical-professionals/other-therapies/