Myasthenia Gravis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
'''Lead Editors''' | '''Lead Editors''' | ||
</div> | </div> | ||
= Historical Aspect | = Historical Aspect<sup></sup> = | ||
The first reported case of MG is likely to be that of the Native American Chief Opechancanough, who died in 1664. It was described by historical chroniclers from Virginia as “the excessive fatigue he encountered wrecked his constitution; his flesh became macerated; the sinews lost their tone and elasticity; and his eyelids were so heavy that he could not see unless they were lifted up by his attendants… he was unable to walk; but his spirit rising above the ruins of his body directed from the litter on which he was carried by his Indians”. In 1672, the English physician Willis first described a patient with “fatigable weakness” involving ocular and bulbar muscles described by his peers as “spurious palsy.” In 1877, Wilks (Guy’s Hospital, London) described the case of a young girl after pathological examination as “bulbar paralysis, fatal, no disease found". In 1879, Wilhelm Erb (Heidelberg, Germany) described three cases of myasthenia gravis in the first paper dealing entirely with this disease, whilst bringing attention to features of bilateral ptosis, diplopia, dysphagia, facial paresis, and weakness of neck muscles. In 1893, Samuel Goldflam (Warsaw, Poland) described three cases with complete description of myasthenia and also analyzed the varying presentations, severity, and prognosis of his cases. Due to significant contributions of Wilhelm Erb and later of Samuel Goldflam, the disease was briefly known as “Erb’s disease” and later for a brief time, it was called “Erb-Goldflam syndrome”. | The first reported case of MG is likely to be that of the Native American Chief Opechancanough, who died in 1664. It was described by historical chroniclers from Virginia as “the excessive fatigue he encountered wrecked his constitution; his flesh became macerated; the sinews lost their tone and elasticity; and his eyelids were so heavy that he could not see unless they were lifted up by his attendants… he was unable to walk; but his spirit rising above the ruins of his body directed from the litter on which he was carried by his Indians”. In 1672, the English physician Willis first described a patient with “fatigable weakness” involving ocular and bulbar muscles described by his peers as “spurious palsy.” In 1877, Wilks (Guy’s Hospital, London) described the case of a young girl after pathological examination as “bulbar paralysis, fatal, no disease found". In 1879, Wilhelm Erb (Heidelberg, Germany) described three cases of myasthenia gravis in the first paper dealing entirely with this disease, whilst bringing attention to features of bilateral ptosis, diplopia, dysphagia, facial paresis, and weakness of neck muscles. In 1893, Samuel Goldflam (Warsaw, Poland) described three cases with complete description of myasthenia and also analyzed the varying presentations, severity, and prognosis of his cases. Due to significant contributions of Wilhelm Erb and later of Samuel Goldflam, the disease was briefly known as “Erb’s disease” and later for a brief time, it was called “Erb-Goldflam syndrome”. | ||
| Line 32: | Line 32: | ||
In Myasthenia gravis (MG) antibodies form against nicotinic acetylcholine (ACh) postsynaptic receptors at the neuromuscular junction (NMJ) of the skeletal muscles<ref>Strauss AJL, Seigal BC, Hsu KC. Immunofluorescence demonstration of a muscle binding complement fixing serum globulin fraction in Myasthenia Gravis. Proc Soc Exp Biol. 1960;105:184</ref>.The basic pathology is a reduction in the number of ACh receptors (AChRs) at the postsynaptic muscle membrane brought about by an acquired autoimmune reaction producing anti-AChR antibodies<ref>Patric J, Lindstrom JM. Autoimmune response to acetylcholine receptor. Science. 1973;180:871</ref>.<br> | In Myasthenia gravis (MG) antibodies form against nicotinic acetylcholine (ACh) postsynaptic receptors at the neuromuscular junction (NMJ) of the skeletal muscles<ref>Strauss AJL, Seigal BC, Hsu KC. Immunofluorescence demonstration of a muscle binding complement fixing serum globulin fraction in Myasthenia Gravis. Proc Soc Exp Biol. 1960;105:184</ref>.The basic pathology is a reduction in the number of ACh receptors (AChRs) at the postsynaptic muscle membrane brought about by an acquired autoimmune reaction producing anti-AChR antibodies<ref>Patric J, Lindstrom JM. Autoimmune response to acetylcholine receptor. Science. 1973;180:871</ref>.<br> | ||
'''NMJ findings that influence susceptibility to muscle weakness and MG:''' EPP generated in normal NMJ is larger than the threshold needed to generate the postsynaptic action potential by a measure of multiple folds. This neuromuscular transmission “safety factor” is reduced in MG patients. Reduction in number or activity of the AChR molecules at the NMJ decreases the EPP, which may be adequate at rest; but when the quantal release of ACh is reduced after repetitive activity, the EPP may fall below the threshold needed to trigger the action potential. This translates as clinical muscle weakness, and when EPP, at rest is consistently below the action potential threshold, it leads to persistent weakness. | '''NMJ findings that influence susceptibility to muscle weakness and MG:''' EPP generated in normal NMJ is larger than the threshold needed to generate the postsynaptic action potential by a measure of multiple folds. This neuromuscular transmission “safety factor” is reduced in MG patients. Reduction in number or activity of the AChR molecules at the NMJ decreases the EPP, which may be adequate at rest; but when the quantal release of ACh is reduced after repetitive activity, the EPP may fall below the threshold needed to trigger the action potential. This translates as clinical muscle weakness, and when EPP, at rest is consistently below the action potential threshold, it leads to persistent weakness.<ref name="7">historical aspect</ref> | ||
*'''Effector Mechanisms of Anti-AChR Antibodies (Anti-AChR Abs)-''' | *'''Effector Mechanisms of Anti-AChR Antibodies (Anti-AChR Abs)-''' | ||
| Line 87: | Line 87: | ||
The sensitivity of this test is approximately 85% for gMG and 50% for oMG. Anti-AChR antibody concentrations cannot be used to predict the severity of disease in individual patients since the concentration of the antibodies does not correlate with the clinical picture. Seronegativity may occur with immunosuppression or if the test is done too early in the disease. As indicated above, striated muscle antibodies against muscle cytoplasmic proteins (titin, myosin, actin, and ryanodine receptors) are detected mainly in patients with thymomatous MG and also in some thymoma patients without MG. The presence of these antibodies in early-onset MG raises the suspicion of a thymoma. Titin antibodies and other striated muscle antibodies are also found in up to 50% of patients with late-onset and nonthymomatous MG and are less helpful as predictors of thymoma in patients over 50 years. Anti-KCNA4 antibodies might be a useful marker to identify patients with thymoma but can be also seen in myocarditis/myositis. Patients with gMG who are anti-AChR antibody negative should be tested for anti-MuSK antibodies which are found in approximately 40% of patients in this group. As noted, low-affinity anti-AChR antibodies binding to clustered AChRs have been found in 66% of sera from patients with seronegative gMG. Whether low-affinity antibodies are present in oMG remains to be determined, but this cell-based assay might eventually provide a more sensitive diagnostic test in this subgroup. Chest CT or MRI is done in all patients with confirmed MG to exclude the presence of a thymoma. Iodinated contrast agents should be used with caution because they might exacerbate myasthenic weakness. MG often coexists with thyroid disease, so baseline testing of thyroid function should be obtained at the time of diagnosis. | The sensitivity of this test is approximately 85% for gMG and 50% for oMG. Anti-AChR antibody concentrations cannot be used to predict the severity of disease in individual patients since the concentration of the antibodies does not correlate with the clinical picture. Seronegativity may occur with immunosuppression or if the test is done too early in the disease. As indicated above, striated muscle antibodies against muscle cytoplasmic proteins (titin, myosin, actin, and ryanodine receptors) are detected mainly in patients with thymomatous MG and also in some thymoma patients without MG. The presence of these antibodies in early-onset MG raises the suspicion of a thymoma. Titin antibodies and other striated muscle antibodies are also found in up to 50% of patients with late-onset and nonthymomatous MG and are less helpful as predictors of thymoma in patients over 50 years. Anti-KCNA4 antibodies might be a useful marker to identify patients with thymoma but can be also seen in myocarditis/myositis. Patients with gMG who are anti-AChR antibody negative should be tested for anti-MuSK antibodies which are found in approximately 40% of patients in this group. As noted, low-affinity anti-AChR antibodies binding to clustered AChRs have been found in 66% of sera from patients with seronegative gMG. Whether low-affinity antibodies are present in oMG remains to be determined, but this cell-based assay might eventually provide a more sensitive diagnostic test in this subgroup. Chest CT or MRI is done in all patients with confirmed MG to exclude the presence of a thymoma. Iodinated contrast agents should be used with caution because they might exacerbate myasthenic weakness. MG often coexists with thyroid disease, so baseline testing of thyroid function should be obtained at the time of diagnosis. | ||
<br> | <br> | ||
== Outcome Measures == | == Outcome Measures == | ||
| Line 105: | Line 105: | ||
#'''Nonsteroidal Immunosuppressive Agents:''' Azathioprine, a purine analog, reduces nucleic acid synthesis, thereby interfering with T-and B-cell proliferation. It has been utilized as an immunosuppressant agent in MG since the 1970s and is effective in 70%–90% of patients with MG. It usually takes up to 15 months to detect clinical response. When used in combination with prednisone, it might be more effective and better tolerated than prednisone alone. Adverse side effects include hepatotoxicity and leukopenia.'''''Mycophenolate''''' mofetil selectively blocks purine synthesis, thereby suppressing both T-cell and B-cell proliferation. Widely used in the treatment of MG, its efficacy in MG was actually suggested by a few non-randomized clinical trial. The standard dose used in MG is 1000 mg twice daily, but doses up to 3000 mg daily can be used. Higher doses are associated with myelosuppression, and complete blood counts should be monitored at least once monthly. The drug is contraindicated in pregnancy and should be used with caution in renal diseases, GI diseases, bone marrow suppression, and elderly patients. '''''Cyclophosphamide'''''administered intravenously and orally is an effective treatment for MG. More than half of the patients become asymptomatic within 1 year of treatment. Undesirable side effects include hair loss, nausea, vomiting, anorexia, and skin discoloration, which limit its use to the management of patients who do not respond to other immunosuppressive treatments. '''''Cyclosporine''''' blocks the synthesis of IL-2 cytokine receptors and other proteins critical to the function of CD4+ T cells. Cyclosporin is used mainly in patients who do not tolerate or respond to azathioprine. Large retrospective studies have supported its use as a steroid-sparing agent.'''''Tacrolimus'''''has been used successfully to treat MG at low doses. It has the theoretical advantage of less nephrotoxicity than cyclosporine. However, there are more controlled trial data supporting the use of cyclosporine. Like other immunosuppressive agents, Tacrolimus also has the potential for severe side effects. MG patients resistant to therapy have been successfully treated with cyclophosphamide in combination with bone marrow transplant or with rituximab, a '''monoclonal antibody''' against the B cell surface marker CD20. '''Etanercept''', a soluble and a recombinant tumor necrosis factor (TNF) receptor blocker, has also been shown to have steroid-sparing effects in studies on small groups of patients. | #'''Nonsteroidal Immunosuppressive Agents:''' Azathioprine, a purine analog, reduces nucleic acid synthesis, thereby interfering with T-and B-cell proliferation. It has been utilized as an immunosuppressant agent in MG since the 1970s and is effective in 70%–90% of patients with MG. It usually takes up to 15 months to detect clinical response. When used in combination with prednisone, it might be more effective and better tolerated than prednisone alone. Adverse side effects include hepatotoxicity and leukopenia.'''''Mycophenolate''''' mofetil selectively blocks purine synthesis, thereby suppressing both T-cell and B-cell proliferation. Widely used in the treatment of MG, its efficacy in MG was actually suggested by a few non-randomized clinical trial. The standard dose used in MG is 1000 mg twice daily, but doses up to 3000 mg daily can be used. Higher doses are associated with myelosuppression, and complete blood counts should be monitored at least once monthly. The drug is contraindicated in pregnancy and should be used with caution in renal diseases, GI diseases, bone marrow suppression, and elderly patients. '''''Cyclophosphamide'''''administered intravenously and orally is an effective treatment for MG. More than half of the patients become asymptomatic within 1 year of treatment. Undesirable side effects include hair loss, nausea, vomiting, anorexia, and skin discoloration, which limit its use to the management of patients who do not respond to other immunosuppressive treatments. '''''Cyclosporine''''' blocks the synthesis of IL-2 cytokine receptors and other proteins critical to the function of CD4+ T cells. Cyclosporin is used mainly in patients who do not tolerate or respond to azathioprine. Large retrospective studies have supported its use as a steroid-sparing agent.'''''Tacrolimus'''''has been used successfully to treat MG at low doses. It has the theoretical advantage of less nephrotoxicity than cyclosporine. However, there are more controlled trial data supporting the use of cyclosporine. Like other immunosuppressive agents, Tacrolimus also has the potential for severe side effects. MG patients resistant to therapy have been successfully treated with cyclophosphamide in combination with bone marrow transplant or with rituximab, a '''monoclonal antibody''' against the B cell surface marker CD20. '''Etanercept''', a soluble and a recombinant tumor necrosis factor (TNF) receptor blocker, has also been shown to have steroid-sparing effects in studies on small groups of patients. | ||
=== Surgical Management (Thymectomy) === | === Surgical Management (Thymectomy) === | ||
<br>Surgical treatment is strongly recommended for patients with thymoma. The clinical efficacy of thymectomy in other situations has been questioned because the evidence supporting its use is not solid. Surgical treatment is strongly recommended for patients with thymoma. The benefit of thymectomy evolves over several years. Thymectomy is advised as soon as the patient’s degree of weakness is sufficiently controlled to permit surgery. Patients undergoing surgery are usually pretreated with low-dose glucocorticoids and IVIg. Thymectomy may not be a viable therapeutic approach for anti-MuSK antibody-positive patients because their thymi lack the germinal centers and infiltrates of lymphocytes that characterize thymi in patients who have anti-AChR antibodies. This supports a different pathologic mechanism in anti-MuSK Ab-positive and anti-AChR Ab-positive MG. Most experts consider thymectomy to be a therapeutic option in anti-AChR Ab-positive gMG with disease onset before the age of 50 years | <br>Surgical treatment is strongly recommended for patients with thymoma. The clinical efficacy of thymectomy in other situations has been questioned because the evidence supporting its use is not solid. Surgical treatment is strongly recommended for patients with thymoma. The benefit of thymectomy evolves over several years. Thymectomy is advised as soon as the patient’s degree of weakness is sufficiently controlled to permit surgery. Patients undergoing surgery are usually pretreated with low-dose glucocorticoids and IVIg. Thymectomy may not be a viable therapeutic approach for anti-MuSK antibody-positive patients because their thymi lack the germinal centers and infiltrates of lymphocytes that characterize thymi in patients who have anti-AChR antibodies. This supports a different pathologic mechanism in anti-MuSK Ab-positive and anti-AChR Ab-positive MG. Most experts consider thymectomy to be a therapeutic option in anti-AChR Ab-positive gMG with disease onset before the age of 50 years | ||
=== Physiotherapy Management === | === Physiotherapy Management === | ||
Revision as of 12:54, 14 January 2015
Original Editor - Wendy Walker
Lead Editors
Historical Aspect[edit | edit source]
The first reported case of MG is likely to be that of the Native American Chief Opechancanough, who died in 1664. It was described by historical chroniclers from Virginia as “the excessive fatigue he encountered wrecked his constitution; his flesh became macerated; the sinews lost their tone and elasticity; and his eyelids were so heavy that he could not see unless they were lifted up by his attendants… he was unable to walk; but his spirit rising above the ruins of his body directed from the litter on which he was carried by his Indians”. In 1672, the English physician Willis first described a patient with “fatigable weakness” involving ocular and bulbar muscles described by his peers as “spurious palsy.” In 1877, Wilks (Guy’s Hospital, London) described the case of a young girl after pathological examination as “bulbar paralysis, fatal, no disease found". In 1879, Wilhelm Erb (Heidelberg, Germany) described three cases of myasthenia gravis in the first paper dealing entirely with this disease, whilst bringing attention to features of bilateral ptosis, diplopia, dysphagia, facial paresis, and weakness of neck muscles. In 1893, Samuel Goldflam (Warsaw, Poland) described three cases with complete description of myasthenia and also analyzed the varying presentations, severity, and prognosis of his cases. Due to significant contributions of Wilhelm Erb and later of Samuel Goldflam, the disease was briefly known as “Erb’s disease” and later for a brief time, it was called “Erb-Goldflam syndrome”.
In 1895, Jolly, at the Berlin Society meeting, described two cases under the title of “myasthenia gravis pseudo-paralytica”. The first two words of this syndrome gradually got accepted as the formal name of this disorder. He also demonstrated a phenomenon, that later came to be known as “Mary Walker effect” after she herself observed and described the same finding in 1938. This was reported as “if you stimulate one group of muscles to exhaustion, weakness is apparent in muscles that are not stimulated; an evidence of a circulating factor causing neuromuscular weakness”
In 1934, Mary Walker realized that MG symptoms were similar to those of curare poisoning, which was treated with physostigmine, a cholinesterase inhibitor. She demonstrated that physostigmine promptly improved myasthenic symptoms. In 1937, Blalock reported improvement in myasthenic patients after thymectomy. Following these discoveries, cholinesterase inhibitor therapy and thymectomy became standard and accepted forms of treatment for MG.
In 1959-1960, Nastuk et al. and Simpson independently proposed that MG has autoimmune etiology. In 1973, Patrick and Lindstrom were able to induce experimental autoimmune MG (EAMG) in a rabbit model using muscle-like acetylcholine receptor (AChR) immunization. In the 1970s prednisone and azathioprine were introduced as treatment modalities for MG followed by plasma exchange that was introduced for acute treatment of severe MG, all supporting the autoimmune etiology.
Definition[edit | edit source]
Myasthenia Gravis is a relatively rare anautoimmune neuromuscular disease leading to fluctuating muscle weakness and fatigue. Muscle weakness is caused by circulating antibodies that block acetylcholine receptors at the postsynaptic neuromuscular junction,inhibiting the excitatory effects of the neurotransmitter acetylcholine on nicotinic receptors at neuromuscular junctions.[1]
Clinically Relevant Anatomy
[edit | edit source]
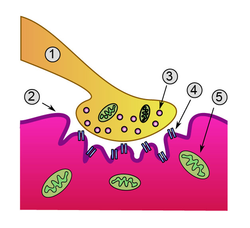
Detailed view of a neuromuscular junction
1. Presynaptic terminal
2. Sarcolemma
3. Synaptic vesicle
4. Nicotinic acetylcholine receptor
5. Mitochondrion
Mechanism of Injury / Pathological Process
[edit | edit source]
In Myasthenia gravis (MG) antibodies form against nicotinic acetylcholine (ACh) postsynaptic receptors at the neuromuscular junction (NMJ) of the skeletal muscles[2].The basic pathology is a reduction in the number of ACh receptors (AChRs) at the postsynaptic muscle membrane brought about by an acquired autoimmune reaction producing anti-AChR antibodies[3].
NMJ findings that influence susceptibility to muscle weakness and MG: EPP generated in normal NMJ is larger than the threshold needed to generate the postsynaptic action potential by a measure of multiple folds. This neuromuscular transmission “safety factor” is reduced in MG patients. Reduction in number or activity of the AChR molecules at the NMJ decreases the EPP, which may be adequate at rest; but when the quantal release of ACh is reduced after repetitive activity, the EPP may fall below the threshold needed to trigger the action potential. This translates as clinical muscle weakness, and when EPP, at rest is consistently below the action potential threshold, it leads to persistent weakness.Cite error: Invalid <ref> tag; name cannot be a simple integer. Use a descriptive title
- Effector Mechanisms of Anti-AChR Antibodies (Anti-AChR Abs)-
Anti-AChR Abs affect neuromuscular transmission by at least 3 mechanisms:
(i) complement binding and activation at the NMJ,
(ii) antigenic modulation (accelerated AChR endocytosis of molecules cross-linked by antibodies),
(iii) functional AChR block—preventing normal ACh to attach and act on AChR.
- Role of CD4+ T Cells in MG-
Pathogenic anti-AChR Abs are high-affinity IgGs-and their synthesis requires activated CD4+ T cells to interact with and stimulate B cells. Therefore, thymectomy, with resultant removal of AChR-specific CD4+ T cells, helps alleviate symptoms in MG patients. Similarly, treatment with anti-CD4+ antibodies has also been shown to have a therapeutic impact. AIDS patients with reduction in CD4+ T cells notice myasthenic symptom improvement.
- Role of CD4+ T-Cell Subtypes and Cytokines in MG and EAMG (Experimental Autoimmune MG)-
CD4+ T cells are classified into two main subtypes: Th1 and Th2 cells. Th1 cells secrete proinflammatory cytokines, such as IL-2, IFN-γ, and TNF-α, which are important in cell-mediated immune responses. Th2 cells secrete anti-inflammatory cytokines, like IL-4, IL-6, and IL-10, which are important inducers of humoral immune responses. IL-4 further stimulates differentiation of Th3 cells that secrete TGF-β, which is involved in immunosuppressive mechanisms.
MG patients have abundant anti-AChR Th1 cells in the blood that recognize many AChR epitopes and are capable of inducing B cells to produce high-affinity anti-AChR antibodies. Th1 cells are indispensible in the development of EAMG as proven in animal models. Therapies against Th1 cytokines (TNF-α and IFN-γ) have been proven in animal models to improve EAMG symptoms.
Anti-AChR Th2 cells have a complex role in EAMG pathogenesis. They can be protective, but their cytokines IL-5, IL-6, and IL-10 may also facilitate EAMG development. CD4+ T cells that express CD25 marker and transcription factor Foxp3 are called “Tregs” and are important in maintaining self-tolerance. Tregs in MG patients may be functionally impaired and are shown to increase after thymectomy with correlated symptom improvement. Role of natural killer (NK) and natural killer T (NKT) cells in MG and EAMG: Natural killer T (NKT) cells with Tregs help in regulating anti-AChR response. Mouse models have shown inhibition of EAMG development after stimulation of NKT cells. IL-18-secreted by antigen-presenting cells (APCs), stimulates NK cells to produce IFN-γ, which permits and enhances Th1 cells to induce EAMG. IL-18-deficient mice are resistant to EAMG, and pharmacologic block of IL-18 suppresses EAMG. MG patients have been shown to have increased serum level of IL-18, which tends to decrease with clinical improvement.
- Other Autoantigens in MG-
Seronegative MG patients (who lack Anti-AChR antibodies) may have anti-MuSK antibodies (up to 40% of this subgroup). Other ethnic groups or locations (e.g., Chinese and Norwegians) have lower frequencies of anti-MuSK antibodies G in seronegative MG patients. MG patients with anti-MuSK antibodies do not have anti-AChR Abs, except as reported in a group of Japanese patients.
Agrin/MuSK signaling pathway maintains the structural and functional integrity of the postsynaptic NMJ apparatus in the adult muscle cell. Anti-MuSK antibodies affect the agrin-dependent AChR cluster maintenance at the NMJ, leading to reduced AChR numbers. Complement-mediated damage may also be responsible for decreasing the AChR numbers at the NMJ when targeted by anti-MuSK Abs. Some human muscle cell culture studies have shown cell cycle arrest, downregulation of AChR subunit with rapsyn, and other muscle protein expression, on exposure to sera from anti-MuSK-positive MG patients. Other antimuscle cell protein antibodies (e.g., antititin and antiryanodine receptor antibodies) are also postulated to have pathogenic roles in MG as discussed earlier.
Clinical Presentation[edit | edit source]
The usual initial complaint is a specific muscle weakness rather than generalized weakness - frequently ocular (eye) symptoms.
Extraocular muscle weakness or ptosis is present initially in 50% of patients, and occurs during the course of illness in 90% of patients.Patients also frequently report diploplia (double vision).
The disease remains exclusively ocular in 10 - 40% of patients.
Rarely, patients have generalized weakness without ocular muscle weakness.
Bulbar muscle weakness is also common, along with weakness of head extension and flexion.
Limb weakness may be more severe proximally than distally.
Isolated limb muscle weakness is the presenting symptom in fewer than 10% of patients.
Weakness is typically least severe in the morning and worsens as the day progresses.
Weakness is increased by exertion and alleviated by rest.
Weakness progresses from mild to more severe over weeks or months, with exacerbations and remissions.
Weakness tends to spread from the ocular to facial to bulbar muscles and then to truncal and limb muscles.
About 87% of patients have generalized disease within 13 months after onset.
Less often, symptoms may remain limited to the extraocular and eyelid muscles for many years.
Classification[edit | edit source]
- Subtypes of MG are broadly classified as follows:
- early-onset MG: age at onset <50 years. Thymic hyperplasia, usually females,
- late-onset MG: age at onset >50 years. Thymic atrophy, mainly males,
- thymoma-associated MG (10%–15%)
- MG with anti-MUSK antibodies,
- ocular MG (oMG): symptoms only affecting extraocular muscles,
- MG with no detectable AChR and muscle-specific tyrosine kinase (MuSK) antibodies.
- The most widely utilised classification of MG is the Myasthenia Gravis Foundation of America Clinical Classification[4]
Class I: Any ocular muscle weakness, possible ptosis, no other evidence of muscle weakness elsewhere
Class II: Mild weakness affecting other than ocular muscles; may also have ocular muscle weakness of any severity
Class IIa: Predominantly affecting limb, axial muscles, or both; may also have lesser involvement of oropharyngeal muscles
Class IIb: Predominantly bulbar and/or respiratory muscles; may also have lesser or equal involvement of limb, axial muscles, or both
Class III: Moderate weakness affecting other than ocular muscles; may also have ocular muscle weakness of any severity
Class IIIa: Predominantly affecting limb, axial muscles, or both; may also have lesser involvement of oropharyngeal muscles
Class IIIb: Predominantly bulbar and/or respiratory muscles; may also have lesser or equal involvement of limb, axial muscles, or both
Class IV: Severe weakness affecting other than ocular muscles; may also have ocular muscle weakness of any severity
Class IVa: Predominantly affecting limb, axial muscles, or both; may also have lesser involvement of oropharyngeal muscles
Class IVb: Predominantly bulbar and/or respiratory muscles; may also have lesser or equal involvement of limb, axial muscles, or both (Can also include feeding tube without intubation)
Class V: Intubation needed to maintain airway, with or without mechanical ventilation
Diagnostic Procedures[edit | edit source]
- Tensilon (Edrophonium Chloride) Test: Edrophonium chloride is a short-acting acetylcholinesterase inhibitor that prolongs the duration of action of acetylcholine at the NMJ. Edrophonium is administered intravenously and the patient is observed for objective improvement in muscle strength particularly the eyelid ptosis and/or extraocular muscle movement. Only unequivocal improvement in strength of a sentinel muscle should be accepted as a positive result. Patients must be connected to cardiac and blood pressure monitors prior to injection because of possible risk of arrhythmia and hypotension. Atropine should be available at bed side for use if an adverse event like severe bradycardia (heart rate below 37) develops. Side effects from Edrophonium include increased salivation and sweating, nausea, stomach cramping, and muscle fasciculation. Hypotension and bradycardia are infrequent and generally resolve with rest in the supine position. Tensilon test has a sensitivity of 71.5%–95% for the diagnosis of MG.
- Ice Pack Test: The ice pack test is a non pharmacological test which could be considered in patients with ptosis when the Edrophonium test is contraindicated. It is performed by placing an ice pack over the eye for 2–5 minutes and assessing for improvement in ptosis.
- Electrophysiological Tests: The two principal electrophysiologic tests for the diagnosis of MG are repetitive nerve stimulation study and single fiber electromyography.
i)Repetitive nerve stimulation tests neuromuscular transmission. It is performed by stimulating the nerve supramaximally at 2-3 Hz. A 10% decrement between the first and the fifth evoked muscle action potential is diagnostic for MG. In the absence of the decrement, exercise can be used to induce exhaustion of muscles and document decrement. The test is abnormal in approximately 75% of patients with gMG and 50% of patients with oMG.
ii)Single-fiber electromyography (SFEMG) is the most sensitive diagnostic test for MG. It is done by using a special needle electrode that allows identification of action potentials from individual muscle fibers. It allows simultaneous recording of the action potentials of two muscle fibers innervated by the same motor axon. The variability in time of the second action potential relative to the first is called “jitter.” In MG, the jitter will increase because the safety factor of transmission at the neuromuscular junction is reduced. SFEMG reveals abnormal jitter in 95%–99% of patients with MG if appropriate muscles are examined. Although highly sensitive, increased jitter is not specific for primary NMJ disease. It may be abnormal in motor neuron disease, polymyositis, peripheral neuropathy, Lambert-Eaton myasthenic syndrome (LEMS), and other neuromuscular disorders. However, it is specific for a disorder of neuromuscular transmission when no other abnormalities are seen on standard needle EMG examination. The most commonly used immunological test for the diagnosis of MG measures the serum concentrations of Anti-AChR antibodies and is highly specific for myasthenia gravis. False positives are rare and may occur with low titers in LEMS (5%), motor neuron disease (3% to 5%), and polymyositis (<1%).
The sensitivity of this test is approximately 85% for gMG and 50% for oMG. Anti-AChR antibody concentrations cannot be used to predict the severity of disease in individual patients since the concentration of the antibodies does not correlate with the clinical picture. Seronegativity may occur with immunosuppression or if the test is done too early in the disease. As indicated above, striated muscle antibodies against muscle cytoplasmic proteins (titin, myosin, actin, and ryanodine receptors) are detected mainly in patients with thymomatous MG and also in some thymoma patients without MG. The presence of these antibodies in early-onset MG raises the suspicion of a thymoma. Titin antibodies and other striated muscle antibodies are also found in up to 50% of patients with late-onset and nonthymomatous MG and are less helpful as predictors of thymoma in patients over 50 years. Anti-KCNA4 antibodies might be a useful marker to identify patients with thymoma but can be also seen in myocarditis/myositis. Patients with gMG who are anti-AChR antibody negative should be tested for anti-MuSK antibodies which are found in approximately 40% of patients in this group. As noted, low-affinity anti-AChR antibodies binding to clustered AChRs have been found in 66% of sera from patients with seronegative gMG. Whether low-affinity antibodies are present in oMG remains to be determined, but this cell-based assay might eventually provide a more sensitive diagnostic test in this subgroup. Chest CT or MRI is done in all patients with confirmed MG to exclude the presence of a thymoma. Iodinated contrast agents should be used with caution because they might exacerbate myasthenic weakness. MG often coexists with thyroid disease, so baseline testing of thyroid function should be obtained at the time of diagnosis.
Outcome Measures[edit | edit source]
add links to outcome measures here (see Outcome Measures Database)
Management / Interventions
[edit | edit source]
Medical Management
[edit | edit source]
(i) symptomatic treatment with acetylcholinesterase inhibitors,
(ii) rapid short-term immunomodulating treatment with plasmapheresis and intravenous immunoglobulin,
(iii) chronic long-term immunomodulating treatment with glucocorticoids and other immunosuppressive drugs,
(iv) surgical treatment.
- Acetylcholinesterase Inhibitors: Acetylcholinesterase inhibitors are the first-line treatment in patients with MG. Response to treatment varies from marked improvement in some patients to little or no improvement in others. Acetylcholinesterase inhibitors are used as a symptomatic therapy and act by increasing the amount of available acetylcholine at the NMJ. They do not alter disease progression or outcome. Pyridostigmine is the most commonly used drug. It has a rapid onset of action within 15 to 30 minutes reaching peak activity in about two hours. The effect lasts for about three to four hours. The initial oral dose is 15–30 mg every 4–6 hours and is titrated upwards depending on the patient’s response. Adverse side effects of Pyridostigmine are mostly due to the cholinergic properties of the drug such as abdominal cramping, diarrhea, increased salivation and bronchial secretions, nausea, sweating, and bradycardia. Nicotinic side effects are also frequent and include muscle fasciculation and cramping. High doses of pyridostigmine exceeding 450 mg daily, administered to patients with renal failure, have been reported to cause worsening of muscle weakness.
- Short-Term Immunomodulating Therapies: Plasma exchange and intravenous immunoglobulin have rapid onset of action with improvement within days, but this is a transient effect. They are used in certain situations such as myasthenic crisis and preoperatively before thymectomy or other surgical procedures. They can be used intermittently to maintain remission in patients with MG who are not well controlled despite the use of chronic immunomodulating drugs.
- Plasmapheresis: It improves strength in most patients with MG by directly removing AChR from the circulation. Typically one exchange is done every other day for a total of four to six times. Adverse effects of plasmapheresis include hypotension, paresthesias, infections, thrombotic complications related to venous access, and bleeding tendencies due to decreased coagulation factors.
- Intravenous Immunoglobulin Therapy (I V Ig): It involves isolating immunoglobulins isolated from pooled human plasma by ethanol cryoprecipitation and is administered for 5 days at a dose of 0.4 g/kg/day, fewer infusions at higher doses are also used. The mechanism of action of IVIg is complex. Factors include inhibition of cytokines competition with autoantibodies, and inhibition of complement deposition. Interference with the binding of Fc receptor on macrophages, Ig receptor on B cells, and interference with antigen recognition by sensitized T cells are other mechanisms. More specific techniques to remove pathogenic anti-AChR antibodies utilizing immunoadsorption have been developed recently, which offer a more targeted approach to MG treatment. Clinical trials showed significant reduction of blocking antibodies with concomitant clinical improvement in patients treated with immunoadsorption techniques.I V Ig is considered to be safe but rare cases of complications do occur such as thrombosis due to increased blood viscosity and other complications related to large volumes of the infused preparation.Compared to plasma exchange, IVIg is similar in terms of efficacy, mortality, and complications. However, plasma exchange (PLEX) has considerable cost advantages over IVIg with a cost benefit ratio of 2 : 1 for treatment of myasthenia gravis.
- Long-Term Immune Therapies: The goal of immune-directed therapy of MG is to induce a remission or near remission of symptoms and maintain it.
- Corticosteroids: These were the first and most commonly used immunosuppressant medications in MG. Prednisone is generally used when symptoms of MG are not adequately controlled by cholinesterase inhibitors alone. Good response can be achieved with initial high doses and then tapering it to the lowest dose to maintain the response. Temporary exacerbation can occur after starting high doses of prednisone within the first 7–10 days which can last for several days. In mild cases, cholinesterase inhibitors are usually used to manage this worsening. In cases known to have severe exacerbations, plasma exchange or IVIg can be given before prednisone therapy to prevent or reduce the severity of corticosteroid-induced weakness and to induce a more rapid response. Oral prednisone might be more effective than anticholinesterase drugs in oMG and should therefore be considered in all patients with oMG.
- Nonsteroidal Immunosuppressive Agents: Azathioprine, a purine analog, reduces nucleic acid synthesis, thereby interfering with T-and B-cell proliferation. It has been utilized as an immunosuppressant agent in MG since the 1970s and is effective in 70%–90% of patients with MG. It usually takes up to 15 months to detect clinical response. When used in combination with prednisone, it might be more effective and better tolerated than prednisone alone. Adverse side effects include hepatotoxicity and leukopenia.Mycophenolate mofetil selectively blocks purine synthesis, thereby suppressing both T-cell and B-cell proliferation. Widely used in the treatment of MG, its efficacy in MG was actually suggested by a few non-randomized clinical trial. The standard dose used in MG is 1000 mg twice daily, but doses up to 3000 mg daily can be used. Higher doses are associated with myelosuppression, and complete blood counts should be monitored at least once monthly. The drug is contraindicated in pregnancy and should be used with caution in renal diseases, GI diseases, bone marrow suppression, and elderly patients. Cyclophosphamideadministered intravenously and orally is an effective treatment for MG. More than half of the patients become asymptomatic within 1 year of treatment. Undesirable side effects include hair loss, nausea, vomiting, anorexia, and skin discoloration, which limit its use to the management of patients who do not respond to other immunosuppressive treatments. Cyclosporine blocks the synthesis of IL-2 cytokine receptors and other proteins critical to the function of CD4+ T cells. Cyclosporin is used mainly in patients who do not tolerate or respond to azathioprine. Large retrospective studies have supported its use as a steroid-sparing agent.Tacrolimushas been used successfully to treat MG at low doses. It has the theoretical advantage of less nephrotoxicity than cyclosporine. However, there are more controlled trial data supporting the use of cyclosporine. Like other immunosuppressive agents, Tacrolimus also has the potential for severe side effects. MG patients resistant to therapy have been successfully treated with cyclophosphamide in combination with bone marrow transplant or with rituximab, a monoclonal antibody against the B cell surface marker CD20. Etanercept, a soluble and a recombinant tumor necrosis factor (TNF) receptor blocker, has also been shown to have steroid-sparing effects in studies on small groups of patients.
Surgical Management (Thymectomy)[edit | edit source]
Surgical treatment is strongly recommended for patients with thymoma. The clinical efficacy of thymectomy in other situations has been questioned because the evidence supporting its use is not solid. Surgical treatment is strongly recommended for patients with thymoma. The benefit of thymectomy evolves over several years. Thymectomy is advised as soon as the patient’s degree of weakness is sufficiently controlled to permit surgery. Patients undergoing surgery are usually pretreated with low-dose glucocorticoids and IVIg. Thymectomy may not be a viable therapeutic approach for anti-MuSK antibody-positive patients because their thymi lack the germinal centers and infiltrates of lymphocytes that characterize thymi in patients who have anti-AChR antibodies. This supports a different pathologic mechanism in anti-MuSK Ab-positive and anti-AChR Ab-positive MG. Most experts consider thymectomy to be a therapeutic option in anti-AChR Ab-positive gMG with disease onset before the age of 50 years
Physiotherapy Management[edit | edit source]
Rehabilitation alone or in combination with other forms of treatment can relieve or reduce symptoms for some people with MG.
MG patients should find the optimal balance between physical activity and rest. It is not possible to cure the weakness by active physical training. However, most MG patients are more passive than they need to be. Physical activity and physical training of low to medium intensity is recommended[5].
One study showed clear benefit from a strength training exercise programme for a group of patients with mild to moderate MG[6], concluding "physical training can be carried out safely in mild MG and provides some improvement of muscle force".
General advice for exercise programmes for people with MG:
- Aim to strengthen large muscle groups, particularly proximal muscles of shoulders and hips
- Advise patient to do the exercises at their "best time of day" ie. when not feeling tired - for the majority of MG patients this will be morning
- If patient is taking pyridostigmine, exercise at peak dose ie. 1.5 to 2 hours after taking a dose
- Moderate intensity of exercise only: patient should not experience worsening of MG symptoms (eg. ptosis or diploplia) during exercise
- General aerobic exercise is also valuable, helping with respiratory function as well stamina
Differential Diagnosis
[edit | edit source]
- Amyotrophic Lateral Sclerosis
- Basilar Artery Thrombosis
- Brainstem Gliomas
- Cavernous Sinus Syndromes
- Dermatomyositis/Polymyositis
- Lambert-Eaton Myasthenic Syndrome
- Multiple Sclerosis
- Myocardial Infarction
- Pulmonary Embolism
- Sarcoidosis and Neuropathy
- Thyroid Disease
- Tolosa-Hunt Syndrome
Key Evidence[edit | edit source]
add text here relating to key evidence with regards to any of the above headings
Resources
[edit | edit source]
The Myasthenia Gravis Foundation of America (MGFA) has a comprehensive website
Case Studies[edit | edit source]
add links to case studies here (case studies should be added on new pages using the case study template)
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ http://en.m.wikipedia.org/wiki/Myasthenia_gravis
- ↑ Strauss AJL, Seigal BC, Hsu KC. Immunofluorescence demonstration of a muscle binding complement fixing serum globulin fraction in Myasthenia Gravis. Proc Soc Exp Biol. 1960;105:184
- ↑ Patric J, Lindstrom JM. Autoimmune response to acetylcholine receptor. Science. 1973;180:871
- ↑ Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. Jul 12 2000;55(1):16-23
- ↑ Skeie GO, Apostolski S, Evoli A et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur. J. Neurol.17,893–902 (2010)
- ↑ Lohi EL1, Lindberg C, Andersen O. Physical training effects in myasthenia gravis. Arch Phys Med Rehabil. 1993 Nov;74(11):1178-80
</div>