Machado-Joseph Disease (Spinocerebellar Ataxia Type 3): Difference between revisions
No edit summary |
Jesse Besaw (talk | contribs) No edit summary |
||
| Line 56: | Line 56: | ||
= Management / Interventions<br> = | = Management / Interventions<br> = | ||
Due to their progressive nature, degenerative cerebellar diseases can be difficult to manage.<sup>[17]</sup> Unlike ataxia following a stroke, which may permit unaffected, intact areas of the cerebellum to compensate for deteriorating areas, degenerative cerebellar diseases begin to affect essentially all parts of the cerebellum. Rehabilitation of motor impairments may also be made difficult because of the cerebellum’s role in motor-learning.<sup>[18]</sup> Thus, low or poor rehabilitative progression indicative of low benefit may be caused by the inability of the damaged cerebellar structures to functionally participate in the relearning of motor skills. Although MJD may be difficult to manage, the cognition of these patients remains intact, making treatment plausible from comprehension perspective. | |||
There is currently no existing modifying treatment for MJD, and therefore, much of the disease management predominantly surrounds the non-motor symptoms. Such symptoms include pain, cramps, fatigue and depression.<sup>[10]</sup> Treatment of the aforementioned symptoms as well as the treatment of sleep disorders, spasticity, parkinsonism, and dystonia must be acknowledged in attempt to improve the quality of life of those affected by MJD.<sup>[4, 10]</sup> | |||
The MJD Foundation illustrates very well what it is like to work with people affected by MJD. This video depicts their clinical approach to management of MJD. | |||
The cerebellum plays a crucial role in the control of an individual’s movement, taking part in the coordination of balance and locomotion.<sup>[19]</sup> Thus, damage to the this region very characteristically translates into a dysfunctional form of movement referred to as gait ataxia. Gait ataxia presents itself as a highly variable type of movement pattern in which the individual has difficulties in maintaining postural balance while walking. Individuals with gait ataxia appear to walk in a “drunk-like” state. | |||
Much of the existing research encompassing coordinative training in degenerative cerebellar diseases is limited to single cases or studies of a small number of patients with varying levels of severity and forms of ataxia.<sup>[17]</sup> For comparative sake, this makes it quite difficult to establish an idea as to what interventions provide the greatest benefit for patients with MJD. However, there is promising evidence suggesting that continuous training can in fact induce functional and stabilizing improvements in degenerative cerebellar diseases.<sup>[17]</sup> | |||
There is evidence that improved postural stability, reduced dependence on walking aids, and increased independence can be achieved with the use of increasingly demanding balance and gait tasks.<sup>[20, 21]</sup> It is believed that postural stability may be improved in patients with MJD by the repetition of activities that challenge their stability.<sup>[21]</sup> In addition, there is evidence that treadmills, both with<sup>[22, 23]</sup> and without<sup>[24]</sup> the use of body-weight support, facilitate locomotion in patients that have more severe forms of ataxia. In some individuals, the benefits of gait training using a treadmill may be further improved when combined with overground gait training.<sup>[22]</sup> Also, dynamic balance in posture and gait as well as intra-limb coordination, in both juvenile and adult patients with degenerative cerebellar diseases, have been found to improve with intensive whole-body coordinative training.<sup>[25, 26]</sup> In fact, continuous coordinative training may reduce ataxic symptoms (ie. gait-like velocity, lateral sway, and intralimb coordination) that not only contribute to improved stability and motor performance but also provide the patient with increased quality of life.<sup>[25]</sup> | |||
It is thought to be true that exercise and physical therapy programs allow patients to better cope with their disabilities, even though there is a lack of evidence suggesting that exercise plays any part in slowing the progression of the disease.<sup>[10]</sup> Much of the coping is believed to come from positive changes in psychological well-being including a greater sense of control over the disease, improved mood, and an increase in one’s self-esteem.<sup>[10]</sup> However, exercise is clinically shown to help with short-term management of symptoms and reduction of comorbidities. | |||
As the risk of falls becomes increasingly apparent with disease progression, it is important that the patient’s safety in day-to-day activity becomes a priority.<sup>[10]</sup> It may be necessary that the patient be evaluated for appropriate physical aids such as a cane, walker or wheelchair and assistive devices that can be implemented at home.<sup>[4, 10]</sup> Implementation of safety measures not only reduce the risk of falls and fractures but also allows the individual to maintain their independence for as long as possible.<sup>[10]</sup> | |||
Much of the pharmacological evidence related to the management of MJD revolves around symptomatic treatment. Although there are a variety of medications that are used to manage a wide range of symptoms, there is a lack of evidence illustrating how these medications specifically affect the MJD population.<sup>[4, 10]</sup> It is important that a physiotherapist be aware that medications may have a significant effect on the rehabilitation process and on the capabilities of the patient during treatment.<sup>[10]</sup> A physiotherapist's role in the assessment of MJD will often come after a referral from a doctor who has diagnosed and cleared the patient for exercise training. It is then the physiotherapist’s job to provide a full evaluation of the individual and create an appropriate exercise program.<sup>[10]</sup> | |||
Acknowledgement of dysarthria and dysphagia via regular speech therapy as well as occupational therapy may also help to manage the disease.<sup>[4, 10]</sup> | |||
Overall, evidence-based management parameters are still rather vague and non-specific, and there is a general consensus that this area of research needs to be improved.<sup>[17]</sup> It would be beneficial if further research was done to differentiate between types of interventions, to determine the particulars of training paramaters, and to provide various approaches to training that correspond to the level of severity of ataxia.<sup>[26]</sup><br> | |||
= Differential Diagnosis<br> = | = Differential Diagnosis<br> = | ||
Revision as of 15:05, 8 May 2017
Clinically Relevant Anatomy[edit | edit source]
Machado- Joseph disease (MJD) or spinocerebellar ataxia type 3 (SCA3) is the most common spinocerebellar ataxia worldwide.[1] MJD can have widespread symptoms due to the the many anatomical structures that can be affected. These structures include:
- Cerebellum (dentate nucleus)[1]
- Basal ganglia (substantia nigra and globus pallidus)[1]
- Midbrain[1]
- Pons[1]
- Thalamus[1]
- Spinal cord (anterior horn and Clarke’s Column)[2]
- Cranial nerves[2]
- Peripheral nerves[1]
Of the aforementioned structures the common finding is degeneration. For example, the encephalon has decreased mass, the cerebellum, pons, medulla oblongata are atrophied and there is loss of neuronal cell bodies in the dentate nucleus, substantia nigra and anterior horn of the spinal cord.[1]
Mechanism of Injury / Pathological Process
[edit | edit source]
MJD is an autosomal dominant inherited disease. This means that a parent who has MJD has a 50% chance of passing down the affected allele to their offspring. Due to the fact that MJD presents its inheritance in a dominant pattern, there cannot be any recessive carriers of the disease. If a person has the affected allele they will always show the symptoms of the disease.[3] A unique feature of MJD and most repeat genetic diseases is called ‘anticipation’. Anticipation is observed when children show an increased progression of the disease with earlier and worse symptoms than their parents. This is thought to occur due to increased repeats of the mutation when passed down from generation to generation.[3]
The affected gene in MJD is named ATXN3. A long and abnormal repeat of the “CAG” code is what causes the disease and the reason for the mutated protein called ataxin-3. This mutation causes the protein to fold abnormally which forms clumps called inclusion bodies in the nucleus of affected central and peripheral nerve cells.[3] The ataxin-3 is found in both neural and non-neural tissue throughout the body and is thought to help with protein quality control pathways. The exact mechanism of the toxic effects of the mutated protein ataxin-3 is presently unknown and the repeated CAG code only partially explains the effects of MJD.[4]
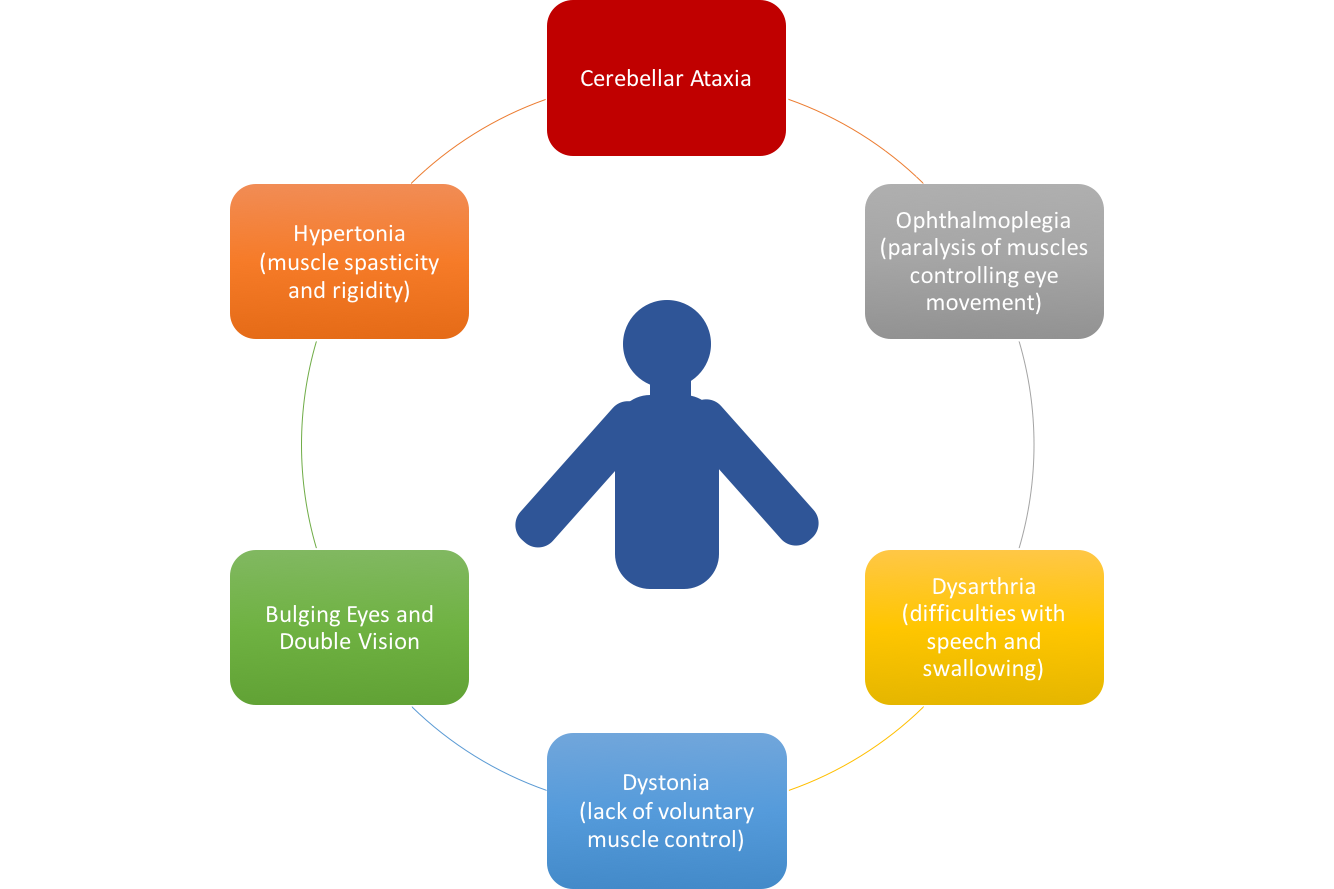
Clinical Presentation
[edit | edit source]
Diagnostic Procedures
[edit | edit source]
Molecular testing for the MJD mutation must be performed to confirm diagnosis.[15] A physiotherapist may refer patient to a doctor when suspecting MJD with observation of symptoms indicating progressive ataxia and pyramidal signs.
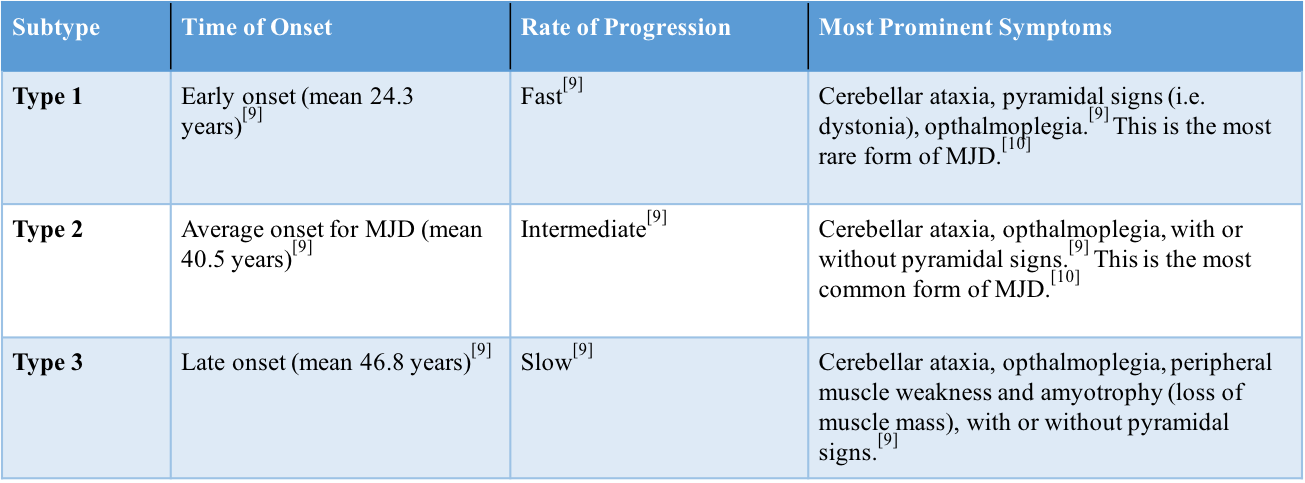
Genetic testing revealing CAG repeats are indicative of MJD diagnosis.[1] The number of these repeats correlates with severity of the disease; increasing number of repeats corresponds with increased reflexes and mortality rate, and vise versa with decreased number of repeats.[12] Type 3 MJD is specifically characterized by fasciculations.[6] Health care practitioners must be cautious to rule out amyotrophic lateral sclerolsis (ALS), as the involvement of motor neurons causes the two conditions to present similarly.[1]
Upon autopsy, the following findings may be present; encephalon of decreased mass, pale-coloured substantia nigra, atrophied cerebellum, medulla oblongata and pons, as well as a decreased number of neuron bodies in the dentate nucleus, substantia nigra and anterior horn of the spinal cord.[1]
Outcome Measures
[edit | edit source]
In order to assess the severity of MJD several scales specific to ataxia can be used. The International Cooperative Ataxia Rating Scale (CARS) and Scale for the Assessment of Rating of Ataxia (SARA) have been shown to be both reliable and effective when used with MJD. (Zhou et al, 2011).
Common assessment measures that are not specific to MJD but may be useful outcome measures for core symptoms include:
- Berg Balance Scale (BBS),
- Romberg Test
- Ashworth Scale
- Tardieu Scale/Modified Tardieu Scale
- Functional Independence Measure (FIM)
- 6 Minute Walk Test
- Time Up and Go (TUG)
- Psychological General Well-being (PGWB) Index
Management / Interventions
[edit | edit source]
Due to their progressive nature, degenerative cerebellar diseases can be difficult to manage.[17] Unlike ataxia following a stroke, which may permit unaffected, intact areas of the cerebellum to compensate for deteriorating areas, degenerative cerebellar diseases begin to affect essentially all parts of the cerebellum. Rehabilitation of motor impairments may also be made difficult because of the cerebellum’s role in motor-learning.[18] Thus, low or poor rehabilitative progression indicative of low benefit may be caused by the inability of the damaged cerebellar structures to functionally participate in the relearning of motor skills. Although MJD may be difficult to manage, the cognition of these patients remains intact, making treatment plausible from comprehension perspective.
There is currently no existing modifying treatment for MJD, and therefore, much of the disease management predominantly surrounds the non-motor symptoms. Such symptoms include pain, cramps, fatigue and depression.[10] Treatment of the aforementioned symptoms as well as the treatment of sleep disorders, spasticity, parkinsonism, and dystonia must be acknowledged in attempt to improve the quality of life of those affected by MJD.[4, 10]
The MJD Foundation illustrates very well what it is like to work with people affected by MJD. This video depicts their clinical approach to management of MJD.
The cerebellum plays a crucial role in the control of an individual’s movement, taking part in the coordination of balance and locomotion.[19] Thus, damage to the this region very characteristically translates into a dysfunctional form of movement referred to as gait ataxia. Gait ataxia presents itself as a highly variable type of movement pattern in which the individual has difficulties in maintaining postural balance while walking. Individuals with gait ataxia appear to walk in a “drunk-like” state.
Much of the existing research encompassing coordinative training in degenerative cerebellar diseases is limited to single cases or studies of a small number of patients with varying levels of severity and forms of ataxia.[17] For comparative sake, this makes it quite difficult to establish an idea as to what interventions provide the greatest benefit for patients with MJD. However, there is promising evidence suggesting that continuous training can in fact induce functional and stabilizing improvements in degenerative cerebellar diseases.[17]
There is evidence that improved postural stability, reduced dependence on walking aids, and increased independence can be achieved with the use of increasingly demanding balance and gait tasks.[20, 21] It is believed that postural stability may be improved in patients with MJD by the repetition of activities that challenge their stability.[21] In addition, there is evidence that treadmills, both with[22, 23] and without[24] the use of body-weight support, facilitate locomotion in patients that have more severe forms of ataxia. In some individuals, the benefits of gait training using a treadmill may be further improved when combined with overground gait training.[22] Also, dynamic balance in posture and gait as well as intra-limb coordination, in both juvenile and adult patients with degenerative cerebellar diseases, have been found to improve with intensive whole-body coordinative training.[25, 26] In fact, continuous coordinative training may reduce ataxic symptoms (ie. gait-like velocity, lateral sway, and intralimb coordination) that not only contribute to improved stability and motor performance but also provide the patient with increased quality of life.[25]
It is thought to be true that exercise and physical therapy programs allow patients to better cope with their disabilities, even though there is a lack of evidence suggesting that exercise plays any part in slowing the progression of the disease.[10] Much of the coping is believed to come from positive changes in psychological well-being including a greater sense of control over the disease, improved mood, and an increase in one’s self-esteem.[10] However, exercise is clinically shown to help with short-term management of symptoms and reduction of comorbidities.
As the risk of falls becomes increasingly apparent with disease progression, it is important that the patient’s safety in day-to-day activity becomes a priority.[10] It may be necessary that the patient be evaluated for appropriate physical aids such as a cane, walker or wheelchair and assistive devices that can be implemented at home.[4, 10] Implementation of safety measures not only reduce the risk of falls and fractures but also allows the individual to maintain their independence for as long as possible.[10]
Much of the pharmacological evidence related to the management of MJD revolves around symptomatic treatment. Although there are a variety of medications that are used to manage a wide range of symptoms, there is a lack of evidence illustrating how these medications specifically affect the MJD population.[4, 10] It is important that a physiotherapist be aware that medications may have a significant effect on the rehabilitation process and on the capabilities of the patient during treatment.[10] A physiotherapist's role in the assessment of MJD will often come after a referral from a doctor who has diagnosed and cleared the patient for exercise training. It is then the physiotherapist’s job to provide a full evaluation of the individual and create an appropriate exercise program.[10]
Acknowledgement of dysarthria and dysphagia via regular speech therapy as well as occupational therapy may also help to manage the disease.[4, 10]
Overall, evidence-based management parameters are still rather vague and non-specific, and there is a general consensus that this area of research needs to be improved.[17] It would be beneficial if further research was done to differentiate between types of interventions, to determine the particulars of training paramaters, and to provide various approaches to training that correspond to the level of severity of ataxia.[26]
Differential Diagnosis
[edit | edit source]
The clinical presentation of MJD can often appear like other neurodegenerative diseases that affect movement, the most common differential diagnoses are:
Key Evidence
[edit | edit source]
Resources
[edit | edit source]
Case Studies
[edit | edit source]
Recent Related Research
[edit | edit source]
References[edit | edit source]
1. Pedroso JL, de Resende Pinto WB, de Souza PV, Andriotti C, Stavale JN, Barsottini OG. Anterior horn degeneration in Machado-Joseph disease. Journal of the Neurological Sciences. 2016 Sep 15;368:290-1.
2. Gilman S. The spinocerebellar ataxias. Clinical neuropharmacology. 2000 Nov 1;23(6):296-303.
3. "Machado-Joseph Disease Fact Sheet | National Institute Of Neurological Disorders And Stroke". Ninds.nih.gov. N.p., 2010. Web. 6 May 2017.
4. Bettencourt, C., & Lima, M. (2011). Machado-Joseph Disease: from first descriptions to new perspectives. Orphanet journal of rare diseases, 6(1), 35.
5. Van de Warrenburg BP, Sinke RJ, Verschuuren–Bemelmans CC, Scheffer H, Brunt ER, Ippel PF, Maat–Kievit JA, Dooijes D, Notermans NC, Lindhout D, Knoers NV. Spinocerebellar ataxias in the Netherlands Prevalence and age at onset variance analysis. Neurology. 2002 Mar 12;58(5):702-8
6. Jardim LB, Pereira ML, Silveira I, Ferro A, Sequeiros J, Giugliani R. Neurologic findings in Machado-Joseph disease: relation with disease duration, subtypes, and (CAG) n. Archives of neurology. 2001 Jun 1;58(6):899-904.
7. Gaspar, C., Lopes-Cendes, I., Hayes, S., Goto, J., Arvidsson, K., Dias, A., ... & Zhou, Y. X. (2001). Ancestral origins of the Machado-Joseph disease mutation: a worldwide haplotype study. The American Journal of Human Genetics, 68(2), 523-528.
8. ADD SOURCE
9. Maciel, P., Gaspar, C., DeStefano, A. L., Silveira, I., Coutinho, P., Radvany, J., ... & Nezarati, M. M. (1995). Correlation between CAG repeat length and clinical features in Machado-Joseph disease. American journal of human genetics, 57(1), 54.
10. D'Abreu A, França MC, Paulson HL, Lopes-Cendes I. Caring for Machado–Joseph disease: current understanding and how to help patients. Parkinsonism & related disorders. 2010 Jan 31;16(1):2-7.
11. Kawai Y, Takeda A, Abe Y, Washimi Y, Tanaka F, Sobue G. Cognitive impairments in Machado-Joseph disease. Archives of neurology. 2004 Nov 1;61(11):1757-60.
12. Durr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, Chneiweiss H, Benomar A, Lyon‐Caen O, Julien J, Serdaru M. Spinocerebellar ataxia 3 and Machado‐Joseph disease: clinical, molecular, and neuropathological features. Annals of neurology. 1996 Apr 1;39(4):490-9.
13. Paulson H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handbook of clinical neurology/edited by PJ Vinken and GW Bruyn. 2012;103:437.
14. Kieling, C., Prestes, P. R., Saraiva‐Pereira, M. L., & Jardim, L. B. (2007). Survival estimates for patients with Machado–Joseph disease (SCA3). Clinical genetics, 72(6), 543-545.
15. Lopes-Cendes I, Silveira I, Maciel P, Gaspar C, Radvany J, Chitayat D, Babul R, Stewart J, Dolliver M, Robitaille Y, Rouleau GA. Limits of clinical assessment in the accurate diagnosis of Machado-Joseph disease. Archives of neurology. 1996 Nov 1;53(11):1168-74.
16. Zhou J, Lei L, Liao X, Wang J, Jiang H, Tang B, Shen L. Related factors of ICARS and SARA scores on spinocerebellar ataxia type 3/Machado-Joseph disease. Zhong nan da xue xue bao. Yi xue ban= Journal of Central South University. Medical sciences. 2011 Jun;36(6):498-503.
17. Ilg W, Bastian AJ, Boesch S, Burciu RG, Celnik P, Claaßen J, Feil K, Kalla R, Miyai I, Nachbauer W, Schöls L. Consensus paper: management of degenerative cerebellar disorders. The Cerebellum. 2014 Apr 1;13(2):248-68.
18. Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Current opinion in neurobiology. 2006 Dec 31;16(6):645-9.
19. Ilg W, Timmann D. Gait ataxia—specific cerebellar influences and their rehabilitation. Movement Disorders. 2013 Sep 15;28(11):1566-75.
20. Balliet R, Harbst KB, Kim D, Stewart RV. Retraining of functional gait through the reduction of upper extremity weight-bearing in chronic cerebellar ataxia. International rehabilitation medicine. 1986 Jan 1;8(4):148-53.
21. Gill-Body KM, Popat RA, Parker SW, Krebs DE. Rehabilitation of balance in two patients with cerebellar dysfunction. Physical therapy. 1997 May 1;77(5):534.
22. Cernak K, Stevens V, Price R, Shumway-Cook A. Locomotor training using body-weight support on a treadmill in conjunction with ongoing physical therapy in a child with severe cerebellar ataxia. Physical Therapy. 2008 Jan 1;88(1):88.
23. Freund JE, Stetts DM. Use of trunk stabilization and locomotor training in an adult with cerebellar ataxia: a single system design. Physiotherapy Theory and Practice. 2010 Oct 1;26(7):447-58.
24. Vaz DV, Schettino RD, Rolla de Castro TR, Teixeira VR, Cavalcanti Furtado SR, de Mello Figueiredo E. Treadmill training for ataxic patients: a single-subject experimental design. Clinical Rehabilitation. 2008 Mar;22(3):234-41.
25. Ilg W, Synofzik M, Brötz D, Burkard S, Giese MA, Schöls L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009 Dec 1;73(22):1823-30.
26. Ilg W, Brötz D, Burkard S, Giese MA, Schöls L, Synofzik M. Long‐term effects of coordinative training in degenerative cerebellar disease. Movement Disorders. 2010 Oct 15;25(13):2239-46.