Molecular Motors: Difference between revisions
No edit summary |
No edit summary |
||
| Line 20: | Line 20: | ||
Two types of specialized motility structures in eucaryotic cells consist of highly ordered arrays of motor proteins that move on stabilized filament tracks. The myosin-actin system of the sarcomere powers the contraction of various types of muscle, including skeletal, smooth, and cardiac muscle. The dynein-microtubule system of the axoneme powers the beating of cilia and the undulations of flagella. | Two types of specialized motility structures in eucaryotic cells consist of highly ordered arrays of motor proteins that move on stabilized filament tracks. The myosin-actin system of the sarcomere powers the contraction of various types of muscle, including skeletal, smooth, and cardiac muscle. The dynein-microtubule system of the axoneme powers the beating of cilia and the undulations of flagella. | ||
== Muscle Contraction == | |||
[[File:Sarcomere.gif|right|frameless|374x374px]] | |||
Muscle contraction is the most familiar and the best understood form of movement in animals. In vertebrates, running, walking, swimming, and flying all depend on the rapid contraction of skeletal muscle on its scaffolding of bone, while involuntary movements such as heart pumping and gut peristalsis depend on the contraction of cardiac muscle and smooth muscle, respectively. All these forms of muscle contraction depend on the ATP-driven sliding of highly organized arrays of actin filaments against arrays of myosin II filaments. | |||
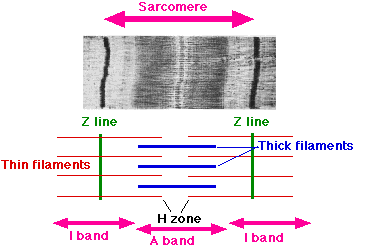
Each sarcomere is formed from a miniature, precisely ordered array of parallel and partly overlapping thin and thick filaments. The thin filaments are composed of actin and associated proteins, and they are attached at their plus ends to a Z disc at each end of the sarcomere. The capped minus ends of the actin filaments extend in toward the middle of the sarcomere, where they overlap with thick filaments, the bipolar assemblies formed from specific muscle isoforms of myosin II | |||
Sarcomere shortening is caused by the myosin filaments sliding past the actin thin filaments, with no change in the length of either type of filament. Bipolar thick filaments walk toward the plus ends of two sets of thin filaments of opposite orientations, driven by dozens of independent myosin heads that are positioned to interact with each thin filament<ref name=":0" />. | |||
== Sub Heading 3 == | == Sub Heading 3 == | ||
Revision as of 07:44, 16 January 2021
Original Editor - Lucinda hampton
Top Contributors - Lucinda hampton and Vidya Acharya
Introduction[edit | edit source]
Did you know inside our bodies are little biological motors that carry cargo inside of our cells? These motors help to move important items inside of our cells. The motors move along tracks inside of our cells, the tracks start at the center of our cells and grow outward. The biological motors move along until they reach the place they need to go. They are vital to our cells dividing and help keep the cells in good working order by moving things inside of our cells.[1]
These fascinating molecular motors called are motor proteins. These proteins bind to a polarized cytoskeletal filament and use the energy derived from repeated cycles of ATP hydrolysis to move steadily along it. Dozens of different motor proteins coexist in every eucaryotic cell.
- They differ in the type of filament they bind to (either actin or microtubules), the direction in which they move along the filament, and the “cargo” they carry.
- Many motor proteins carry membrane-enclosed organelles—such as mitochondria, Golgi stacks, or secretory vesicles—to their appropriate locations in the cell.
- Other motor proteins cause cytoskeletal filaments to slide against each other, generating the force that drives such phenomena as muscle contraction, ciliary beating, and cell division.[2]
Sub Heading 2[edit | edit source]
Motor proteins use the energy of ATP hydrolysis to move along microtubules or actin filaments. They mediate the sliding of filaments relative to one another and the transport of membrane-enclosed organelles along filament tracks.
- All known motor proteins that move on actin filaments are members of the myosin superfamily.
- The motor proteins that move on microtubules are members of either the kinesin superfamily or the dynein family.
The myosin and kinesin superfamilies are diverse, with about 40 genes encoding each type of protein in humans. The only structural element shared among all members of each superfamily is the motor “head” domain. These heads can be attached to a wide variety of “tails,” which attach to different types of cargo and enable the various family members to perform different functions in the cell. Although myosin and kinesin walk along different tracks and use different mechanisms to produce force and movement by ATP hydrolysis, they share a common structural core, suggesting that they are derived from a common ancestor[2].
Two types of specialized motility structures in eucaryotic cells consist of highly ordered arrays of motor proteins that move on stabilized filament tracks. The myosin-actin system of the sarcomere powers the contraction of various types of muscle, including skeletal, smooth, and cardiac muscle. The dynein-microtubule system of the axoneme powers the beating of cilia and the undulations of flagella.
Muscle Contraction[edit | edit source]
Muscle contraction is the most familiar and the best understood form of movement in animals. In vertebrates, running, walking, swimming, and flying all depend on the rapid contraction of skeletal muscle on its scaffolding of bone, while involuntary movements such as heart pumping and gut peristalsis depend on the contraction of cardiac muscle and smooth muscle, respectively. All these forms of muscle contraction depend on the ATP-driven sliding of highly organized arrays of actin filaments against arrays of myosin II filaments.
Each sarcomere is formed from a miniature, precisely ordered array of parallel and partly overlapping thin and thick filaments. The thin filaments are composed of actin and associated proteins, and they are attached at their plus ends to a Z disc at each end of the sarcomere. The capped minus ends of the actin filaments extend in toward the middle of the sarcomere, where they overlap with thick filaments, the bipolar assemblies formed from specific muscle isoforms of myosin II
Sarcomere shortening is caused by the myosin filaments sliding past the actin thin filaments, with no change in the length of either type of filament. Bipolar thick filaments walk toward the plus ends of two sets of thin filaments of opposite orientations, driven by dozens of independent myosin heads that are positioned to interact with each thin filament[2].
Sub Heading 3[edit | edit source]
Resources[edit | edit source]
- bulleted list
- x
or
- numbered list
- x
References[edit | edit source]
- ↑ Penn state Uni.MOLECULAR MOTORS FAQS Available from:https://www.mrsec.psu.edu/content/molecular-motors-faqs (accessed 16.1.2021)
- ↑ 2.0 2.1 2.2 Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular motors. InMolecular Biology of the Cell. 4th edition 2002. Garland Science.Available from: https://www.ncbi.nlm.nih.gov/books/NBK26888/(accessed 16.1.2021)