Referred Pain: Difference between revisions
mNo edit summary |
Kim Jackson (talk | contribs) m (Text replacement - "[[Dry needling" to "[[Dry Needling") |
||
| (14 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
<div class="editorbox"> | <div class="editorbox"> | ||
'''Original Editor-''' [[User:Karsten De Koster|Karsten De Koster]] | |||
'''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}</div> | |||
== Definition/Description == | |||
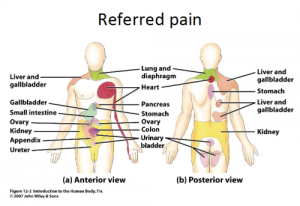
== < | Referred [[Pain Behaviours|pain]] is pain perceived at a location other than the site of the painful stimulus/ origin.<ref name="p1">Arendt-Nielsen L, Svensson P .Referred muscle pain: basic and clinical findings. Clin J Pain, 2001, 17 (1): 11–9.</ref> It is the result of a network of interconnecting sensory nerves, that supplies many different tissues. When there is an injury at one site in the network it is possible that when the signal is interpreted in the [[Brain Anatomy|brain]] signals are experienced in the surrounding nervous tissue. <ref name="p2">Urits I, Burshtein A, Sharma M, Testa L, Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B, Kaye AD. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 2019 Mar 11;23(3):23. doi: 10.1007/s11916-019-0757-1. PMID: 30854609.</ref> | ||
This however should not be confused with radiating or radicular pain which is the pain brought on by anomalous discharges coming from a dorsal root or its ganglion. Radicular pain can be caused by intervertebral disc herniation, [https://www.physio-pedia.com/Spondylolysis Spondylosis], [https://www.physio-pedia.com/Spondylolisthesis Spondylolisthesis], or any condition which involves the compression of the dorsal root ganglion.<ref name=":1">Nikolai Bogduk. On the definitions and physiology of back pain, referred pain, and radicular pain. PAIN 147 (2009) 17–19</ref> | |||
== | == Clinically Relevant Anatomy == | ||
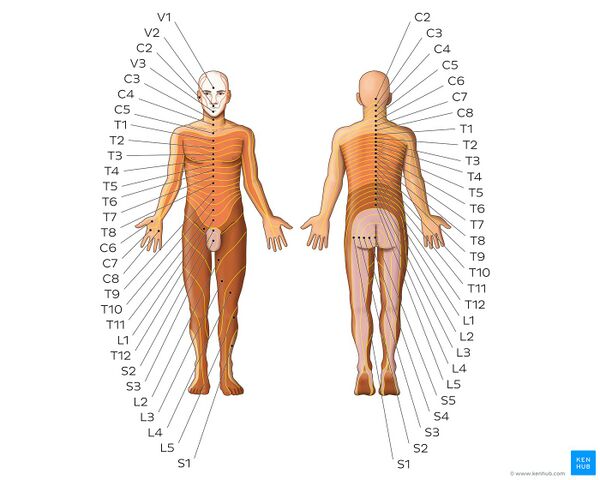
[[File:Dermatomes - Kenhub.jpeg|alt=Dermatomes - Keegan and Garrett map|right|frameless|600x600px|Dermatomes - Keegan and Garrett map]] | |||
Several neuroanatomic and physiologic theories state that nociceptive dorsal horn and [[Brainstem|brain stem]] neurons receive convergent inputs from various tissues. As a result, higher centres cannot correctly identify the actual input source. Recent theories have suggested models in which plasticity of dorsal horn and brainstem neurons play a central role. During the past decade, a systematic attempt to chart referred musculoskeletal pain areas in humans has been made.<ref name="p1" /> | |||
Image: Dermatomes - Keegan and Garrett map<ref > Dermatomes - Keegan and Garrett map image - © Kenhub https://www.kenhub.com/en/study/anatomy-pns-dermatomes</ref> | |||
<br>Nerve fibers of higher | <br>Nerve fibers of higher region sensory inputs such as the skin and nerve fibers of lower sensory inputs such as the stomach converge at the same level of the spinal cord. This can result in confusion on where the sensation/pain is coming from so that stimulus of the lower sensory inputs to the brain can interpreted as coming from the higher regions, resulting in the the pain sensation being located along the related dermatome of the same spinal segment. <ref name="p3">Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci. 2018 Jul 24;19(8):2164. doi: 10.3390/ijms19082164. PMID: 30042373; PMCID: PMC6121522.</ref> | ||
<br> | <br>A prime example of this phenomenon is the pain experienced in cardiac ischemia; the pain is felt in the neck, left shoulder and down the left arm. The referred pain occurs because of multiple primary [[Sensory-Discriminative and Affective-Motivational Components of Pain|sensory]] neurons converging on a single ascending tract. When the painful stimuli arise in visceral receptors the brain is unable to distinguish visceral signals from the more common signals that arise from somatic receptors. This results in pain being interpreted as coming from the somatic regions rather than the viscera.<ref name="p4">Cummings B., Human Physiology, Silverthorn, Pearson, 5the edition, p. 348. </ref> | ||
< | Imman and Saunders suggested that referred pain followed the distribution of sclerotomes (muscle, fascia, and bone) more frequently than it followed the classical dermatomes. Sensory manifestations of clinical and experimental muscle pain are seen as diffuse aching pain in the muscle, pain referred to distant somatic structures, and modifications in superficial and deep tissue sensibility in the painful areas. Hypothetically, convergence of nociceptive afferents on dorsal horn neurons may mediate referred pain.<ref name="p1" /> | ||
[[Image:Tumblr_lb03ir2vgL1qzr2i2o1_500.png|300x300px]] | [[Image:Tumblr_lb03ir2vgL1qzr2i2o1_500.png|300x300px]] | ||
== Neuro-Physiological Theories == | |||
Several neuro-physiological theories have been suggested<ref name="p1" />:<br>• Convergence-projection theory: This states that the pain is caused by convergence of afferent information of the visceral organs and those of somatic origin on the same segment. This causes hyperreactivity of the dorsal horn neurons which is interpreted as coming from the same dermatome.<ref name=":0">Mense S. Neurobiologische Mechanismen der Ubertragung von Muskelschmerz [Neurobiological mechanisms of muscle pain referral.]. Schmerz. 1993 Dec;7(4):241-9. German. doi: 10.1007/BF02529860. PMID: 18415388.</ref><br>• Convergence-facilitation theory: Here, subthreshold sensory input from the lower cutaneous receptors are synapsed upon the same spinothalamic cell body as the sensory input from the sinuvertebral nerve.<ref>Simmons J, Ricketson R, McMillin J. Painful lumbosacral sensory distribution patterns: Embryogenesis to adulthood. Orthopaedic review, 1993, 22(10):1110-8</ref><br>• Axon-reflex theory: The axon reflex is the spread of cutaneous receptor impulse from the main axon to nearby blood vessels in the stimulated area of the skin. These impulses release chemical agents that cause blood vessels to dilate and leak, causing the skin to sweat, and vessels to dilate.<ref>Mevlut Y. The axon reflex" Neuroanatomy: An Annual Interdisciplinary Journal of Neuroanatomy. 2008.Print.</ref> <br>• Hyperexcitabillity theory<ref>Lidbeck J. Central hyperexcitability in chronic musculoskeletal pain: a conceptual breakthrough with multiple clinical implications. Pain Res Manag. 2002 Summer;7(2):81-92. doi: 10.1155/2002/310974. PMID: 12185372.</ref><br>• Thalamic convergence theory<ref name=":0" /> | |||
== Epidemiology /Etiology == | |||
The most common causes of referred pain are pain radiating from: a spinal segment, a sacroiliac joint, viscera, tumors, infections or from associated manifestations.<ref name="p5">Da Silva J.A. Pereira, Woolf Anthony D. (eds.) Rheumatology in Practice. Springer, 2010. — 533 p. — ISBN: 978-1-84882-580-2</ref><ref name="p6">Mehta N, George E. Head, Face, and Neck Pain Science, Evaluation, and Management ; John Wiley & Sons, 20-Sept-2011, p. 534.</ref> | |||
It should also be noted that the pain is always related to the nerve of this particular area. For example when the ninth cranial nerve (glossopharyngeal nerve) is involved the pain is felt deep in the ear. This is in contrast to the more superior located pain when the trigeminal nerve is involved.<ref name="p6" /><br> | |||
== Characteristics/Clinical Presentation == | |||
* The area of referred pain is related to the intensity and duration of ongoing/evoked pain. | |||
* The pain is particularly dull, aching or gnawing, and is sometimes described as an expanding pressure. It spreads out into wide areas, making it challenging to localise.<ref name=":1" /> | |||
* Temporal summation is a potent mechanism for generation of referred muscle pain. | |||
* Central hyperexcitability is important for the extent of referred pain. | |||
* Patients with chronic musculoskeletal pains have enlarged referred pain areas to experimental stimuli. The proximal spread of referred muscle pain is seen in patients with chronic musculoskeletal pain that is very seldom seen in healthy individuals. | |||
* Modality-specific somatosensory changes occur in referred areas, which emphasises the importance of using a multimodal sensory test protocol during assessment.<ref name="p1" /> | |||
* There are no neurological symptoms (e.g numbness and paraesthesia) seen since referred pain is not caused by compression of nerve roots.<ref name=":1" /> | |||
== Diagnostic Procedures == | |||
Studies of clinical pain are limited by bias because of cognitive, emotional, and social aspects of the disease. Pain is a multidimensional and highly individualized perception that is very difficult to quantify and to validate in the clinical setting. In experimental pain, the researchers have the possibility to control the intensity of the stimulus ,its duration and also its modality.<ref name="p1" /> With experimental pain, the psychological evoked response can be assessed qualitatively (using, for example, the [[McGill Pain Questionnaire|McGill]] Pain Questionnaire) or quantitatively (using, for example, [[Visual Analogue Scale|visual]] analogue scores).<ref name="p1" /><br>Endogenous methods are suitable for studying general pain states but not for referred pain as they are characterized by a high response rate. But they have also the disadvantage because they involve several or all muscle groups. Therefore it’s better to use exogenous models.<ref name="p1" /> <br>The exogenous model that is the most used is the intramuscular infusion of hypertonic saline. After the infusion, referred pain will be felt in structures at a distance from the infusion site.<ref name="p1" /> There it will appear with a delay of approximately 20 seconds in comparison with local pain <ref name="p7">Graven-Nielsen T. et al, Stimulus-response functions in areas with experimentally induced referred muscle pain- a psychophysical study, Brain Res, 1997, 744,p. 121-8. </ref>. The patient will experience this pain as being diffuse and unpleasant.<ref name="p8">Graven-Nielsen T. et al, Quantification of local and referred muscle pain in humans after sequential i.m. injections of hypertonic saline, Pain 1997,69, p. 111-7. </ref>The advantages and disadvantages of hypertonic saline are: | |||
Studies of clinical pain are limited by bias because of cognitive, emotional, and social aspects of the disease. Pain is a multidimensional and highly individualized perception that is very difficult to quantify and to validate in the clinical setting. In experimental pain, the researchers have the possibility to control the intensity of the stimulus ,its duration and also its modality.<ref name="p1" /> With experimental pain | |||
<br>Endogenous methods are not | |||
<br>The exogenous model that is the most used is the intramuscular infusion of hypertonic saline. After the infusion, referred pain will be felt in structures at a distance from the infusion site.<ref name="p1" /> There it will appear with a delay of approximately 20 seconds in comparison with local pain <ref name="p7">Graven-Nielsen T. et al, Stimulus-response functions in areas with experimentally induced referred muscle pain- a psychophysical study, Brain Res, 1997, 744,p. 121-8. | |||
{| width="200" cellspacing="1" cellpadding="1" border="1" | {| width="200" cellspacing="1" cellpadding="1" border="1" | ||
|- | |- | ||
| Line 51: | Line 49: | ||
| Disadvantages<br> | | Disadvantages<br> | ||
|- | |- | ||
| | | | ||
*Easy | *Easy | ||
*Safe | *Safe | ||
*Induces local and referred pain in most individuals (40-85%) | *Induces local and referred pain in most individuals (40-85%) | ||
<br> | <br> | ||
| | | | ||
| Line 63: | Line 61: | ||
|} | |} | ||
<br>Also other alogenic substances such as bradykinin, serotonin, capsaicin and substance P can be used to cause referred pain.<ref name="p1" /><ref name="p2" /><ref name="p3" /> | |||
< | Another method to cause referred pain is to use intramuscular electrical stimulation. There is a significantly higher stimulus intensity needed elicit referred pain in comparison with local pain, and there is a significantly positive correlation been found among the stimulus intensity and the local pain and referred pain intensity ratings.<ref name="p1" /><ref name="p4" /> | ||
Results are showing us that there is a significant correlation between the size of local pain and referred pain areas and the local sensation/pain and referred sensation/pain intensity ratings.<ref name="p1" /><ref name="p4" /> Also increased nociceptive input that will go to the dorsal horn or the brainstem neurons, which generates an expansion of receptive fields, may be responsible for the expansion of referred areas that are detected during an increased intramuscular stimulation. <ref name="p4" /> | |||
== Examination == | |||
Several explanations regarding the divergent results obtained when an area of referred pain is anesthetized have been offered <ref name="p4" /><ref name="p1" />: | |||
# The variation in the number of structures (skin, subcutis, fascia, muscle, tendons, ligaments, and bone) that is anesthetized. This is the most important criteria, because referred pain areas and, especially visceral referred pain, are commonly found to be located in the deep tissues in which complete anaesthesia of a referred pain area is difficult.<ref name="p1" /><ref name="p4" /> | |||
# The duration and level of local pain. <ref name="p1" /><ref name="p4" /> | |||
# The site of the local pain (skin, viscera, and deep structures). <ref name="p1" /><ref name="p4" /> | |||
Several explanations regarding the divergent results obtained when an area of referred pain is anesthetized have been offered <ref name="p4" /><ref name="p1" />: | # Whether sensory changes (hypersensitivity) occur at the referred pain site.<ref name="p1" /><ref name="p4" /> | ||
== Medical Management == | == Medical Management == | ||
Several studies have found that the area of the referred pain correlated with the intensity and duration of the muscle pain, which parallels the observations for cutaneous secondary hyperalgesia. <ref name="p6"/><ref name="p5"/> | Several studies have found that the area of the referred pain correlated with the intensity and duration of the muscle pain, which parallels the observations for cutaneous secondary hyperalgesia. <ref name="p6" /><ref name="p5" /> | ||
The most effective treatment for chronic musculoskeletal pain is the using of NMDA-receptor antagonists (ketamin), this gives us better results than using conventional morphine management. <ref name="p1" /><ref name="p5" />.<br>Also the applying of an eutectic mixture of local anesthetia on the skin, just above the referred pain area, reduced the referred pain intensity with 22.7%. <ref name="p6" /> A similar result was found after that ethyl chloride was sprayed onto a saline-induced referred pain area.<ref name="p7" /><br>There are two techniques to block all afferents from the referred pain area:<ref name="p6" /> | |||
# Differential nerve blocking with an inflated tourniquet between the site of stimulation and the corresponding distal referred area | |||
# Intravenous regional analgesia | |||
# By this the referred pain intensity was reduced by 40.2 % | |||
Other ways to treat the pain are: | |||
# [[Acupuncture|Acupuncture]] | |||
# Osteopathic manual medicine techniques | |||
# [[Trigger Points|Trigger point injections]] <ref name="p1" /> | |||
# Laser therapy<ref name="p1" /> | |||
# Superficial dry needling is the most effective in combination with stretching <ref name="p2" /> | |||
== Physical Therapy Management == | |||
Physical Therapy [[Pain-Modulation|management]] in referred pain syndrome will mainly work around [[Pain Mechanisms|pain mechanisms]] and their treatment. It will be majorly symptomatic and change from person to person. A few of the techniques that can be utilized for pain management are<ref name="p1" />: | |||
< | # [[Dry Needling]] | ||
# [[Massage|Massage]] | |||
# Application of heat or ice | |||
# [[Transcutaneous Electrical Nerve Stimulation (TENS)|Transcutaneous electrical nerve stimulation]] | |||
# Ethyl chloride spray and stretch technique | |||
# [[Therapeutic Ultrasound|Ultrasound]] | |||
# Manual methods | |||
# Exercise | |||
== References | == References == | ||
<references /> | <references /> | ||
[[Category:Pain]] | [[Category:Pain]] | ||
[[Category:Conditions]] | |||
[[Category:Physiology]] | |||
Latest revision as of 13:53, 29 January 2024
Original Editor- Karsten De Koster
Top Contributors - Karsten De Koster, Andeela Hafeez, Kim Jackson, Rucha Gadgil, WikiSysop, Joao Costa, 127.0.0.1, Rachael Lowe, Venus Pagare, Kai A. Sigel and David OlukayodeDefinition/Description[edit | edit source]
Referred pain is pain perceived at a location other than the site of the painful stimulus/ origin.[1] It is the result of a network of interconnecting sensory nerves, that supplies many different tissues. When there is an injury at one site in the network it is possible that when the signal is interpreted in the brain signals are experienced in the surrounding nervous tissue. [2]
This however should not be confused with radiating or radicular pain which is the pain brought on by anomalous discharges coming from a dorsal root or its ganglion. Radicular pain can be caused by intervertebral disc herniation, Spondylosis, Spondylolisthesis, or any condition which involves the compression of the dorsal root ganglion.[3]
Clinically Relevant Anatomy[edit | edit source]
Several neuroanatomic and physiologic theories state that nociceptive dorsal horn and brain stem neurons receive convergent inputs from various tissues. As a result, higher centres cannot correctly identify the actual input source. Recent theories have suggested models in which plasticity of dorsal horn and brainstem neurons play a central role. During the past decade, a systematic attempt to chart referred musculoskeletal pain areas in humans has been made.[1]
Image: Dermatomes - Keegan and Garrett map[4]
Nerve fibers of higher region sensory inputs such as the skin and nerve fibers of lower sensory inputs such as the stomach converge at the same level of the spinal cord. This can result in confusion on where the sensation/pain is coming from so that stimulus of the lower sensory inputs to the brain can interpreted as coming from the higher regions, resulting in the the pain sensation being located along the related dermatome of the same spinal segment. [5]
A prime example of this phenomenon is the pain experienced in cardiac ischemia; the pain is felt in the neck, left shoulder and down the left arm. The referred pain occurs because of multiple primary sensory neurons converging on a single ascending tract. When the painful stimuli arise in visceral receptors the brain is unable to distinguish visceral signals from the more common signals that arise from somatic receptors. This results in pain being interpreted as coming from the somatic regions rather than the viscera.[6]
Imman and Saunders suggested that referred pain followed the distribution of sclerotomes (muscle, fascia, and bone) more frequently than it followed the classical dermatomes. Sensory manifestations of clinical and experimental muscle pain are seen as diffuse aching pain in the muscle, pain referred to distant somatic structures, and modifications in superficial and deep tissue sensibility in the painful areas. Hypothetically, convergence of nociceptive afferents on dorsal horn neurons may mediate referred pain.[1]
Neuro-Physiological Theories[edit | edit source]
Several neuro-physiological theories have been suggested[1]:
• Convergence-projection theory: This states that the pain is caused by convergence of afferent information of the visceral organs and those of somatic origin on the same segment. This causes hyperreactivity of the dorsal horn neurons which is interpreted as coming from the same dermatome.[7]
• Convergence-facilitation theory: Here, subthreshold sensory input from the lower cutaneous receptors are synapsed upon the same spinothalamic cell body as the sensory input from the sinuvertebral nerve.[8]
• Axon-reflex theory: The axon reflex is the spread of cutaneous receptor impulse from the main axon to nearby blood vessels in the stimulated area of the skin. These impulses release chemical agents that cause blood vessels to dilate and leak, causing the skin to sweat, and vessels to dilate.[9]
• Hyperexcitabillity theory[10]
• Thalamic convergence theory[7]
Epidemiology /Etiology[edit | edit source]
The most common causes of referred pain are pain radiating from: a spinal segment, a sacroiliac joint, viscera, tumors, infections or from associated manifestations.[11][12]
It should also be noted that the pain is always related to the nerve of this particular area. For example when the ninth cranial nerve (glossopharyngeal nerve) is involved the pain is felt deep in the ear. This is in contrast to the more superior located pain when the trigeminal nerve is involved.[12]
Characteristics/Clinical Presentation[edit | edit source]
- The area of referred pain is related to the intensity and duration of ongoing/evoked pain.
- The pain is particularly dull, aching or gnawing, and is sometimes described as an expanding pressure. It spreads out into wide areas, making it challenging to localise.[3]
- Temporal summation is a potent mechanism for generation of referred muscle pain.
- Central hyperexcitability is important for the extent of referred pain.
- Patients with chronic musculoskeletal pains have enlarged referred pain areas to experimental stimuli. The proximal spread of referred muscle pain is seen in patients with chronic musculoskeletal pain that is very seldom seen in healthy individuals.
- Modality-specific somatosensory changes occur in referred areas, which emphasises the importance of using a multimodal sensory test protocol during assessment.[1]
- There are no neurological symptoms (e.g numbness and paraesthesia) seen since referred pain is not caused by compression of nerve roots.[3]
Diagnostic Procedures[edit | edit source]
Studies of clinical pain are limited by bias because of cognitive, emotional, and social aspects of the disease. Pain is a multidimensional and highly individualized perception that is very difficult to quantify and to validate in the clinical setting. In experimental pain, the researchers have the possibility to control the intensity of the stimulus ,its duration and also its modality.[1] With experimental pain, the psychological evoked response can be assessed qualitatively (using, for example, the McGill Pain Questionnaire) or quantitatively (using, for example, visual analogue scores).[1]
Endogenous methods are suitable for studying general pain states but not for referred pain as they are characterized by a high response rate. But they have also the disadvantage because they involve several or all muscle groups. Therefore it’s better to use exogenous models.[1]
The exogenous model that is the most used is the intramuscular infusion of hypertonic saline. After the infusion, referred pain will be felt in structures at a distance from the infusion site.[1] There it will appear with a delay of approximately 20 seconds in comparison with local pain [13]. The patient will experience this pain as being diffuse and unpleasant.[14]The advantages and disadvantages of hypertonic saline are:
| Advantages |
Disadvantages |
|
|
Also other alogenic substances such as bradykinin, serotonin, capsaicin and substance P can be used to cause referred pain.[1][2][5]
Another method to cause referred pain is to use intramuscular electrical stimulation. There is a significantly higher stimulus intensity needed elicit referred pain in comparison with local pain, and there is a significantly positive correlation been found among the stimulus intensity and the local pain and referred pain intensity ratings.[1][6]
Results are showing us that there is a significant correlation between the size of local pain and referred pain areas and the local sensation/pain and referred sensation/pain intensity ratings.[1][6] Also increased nociceptive input that will go to the dorsal horn or the brainstem neurons, which generates an expansion of receptive fields, may be responsible for the expansion of referred areas that are detected during an increased intramuscular stimulation. [6]
Examination[edit | edit source]
Several explanations regarding the divergent results obtained when an area of referred pain is anesthetized have been offered [6][1]:
- The variation in the number of structures (skin, subcutis, fascia, muscle, tendons, ligaments, and bone) that is anesthetized. This is the most important criteria, because referred pain areas and, especially visceral referred pain, are commonly found to be located in the deep tissues in which complete anaesthesia of a referred pain area is difficult.[1][6]
- The duration and level of local pain. [1][6]
- The site of the local pain (skin, viscera, and deep structures). [1][6]
- Whether sensory changes (hypersensitivity) occur at the referred pain site.[1][6]
Medical Management[edit | edit source]
Several studies have found that the area of the referred pain correlated with the intensity and duration of the muscle pain, which parallels the observations for cutaneous secondary hyperalgesia. [12][11]
The most effective treatment for chronic musculoskeletal pain is the using of NMDA-receptor antagonists (ketamin), this gives us better results than using conventional morphine management. [1][11].
Also the applying of an eutectic mixture of local anesthetia on the skin, just above the referred pain area, reduced the referred pain intensity with 22.7%. [12] A similar result was found after that ethyl chloride was sprayed onto a saline-induced referred pain area.[13]
There are two techniques to block all afferents from the referred pain area:[12]
- Differential nerve blocking with an inflated tourniquet between the site of stimulation and the corresponding distal referred area
- Intravenous regional analgesia
- By this the referred pain intensity was reduced by 40.2 %
Other ways to treat the pain are:
- Acupuncture
- Osteopathic manual medicine techniques
- Trigger point injections [1]
- Laser therapy[1]
- Superficial dry needling is the most effective in combination with stretching [2]
Physical Therapy Management[edit | edit source]
Physical Therapy management in referred pain syndrome will mainly work around pain mechanisms and their treatment. It will be majorly symptomatic and change from person to person. A few of the techniques that can be utilized for pain management are[1]:
- Dry Needling
- Massage
- Application of heat or ice
- Transcutaneous electrical nerve stimulation
- Ethyl chloride spray and stretch technique
- Ultrasound
- Manual methods
- Exercise
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Arendt-Nielsen L, Svensson P .Referred muscle pain: basic and clinical findings. Clin J Pain, 2001, 17 (1): 11–9.
- ↑ 2.0 2.1 2.2 Urits I, Burshtein A, Sharma M, Testa L, Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B, Kaye AD. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 2019 Mar 11;23(3):23. doi: 10.1007/s11916-019-0757-1. PMID: 30854609.
- ↑ 3.0 3.1 3.2 Nikolai Bogduk. On the definitions and physiology of back pain, referred pain, and radicular pain. PAIN 147 (2009) 17–19
- ↑ Dermatomes - Keegan and Garrett map image - © Kenhub https://www.kenhub.com/en/study/anatomy-pns-dermatomes
- ↑ 5.0 5.1 Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci. 2018 Jul 24;19(8):2164. doi: 10.3390/ijms19082164. PMID: 30042373; PMCID: PMC6121522.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 Cummings B., Human Physiology, Silverthorn, Pearson, 5the edition, p. 348.
- ↑ 7.0 7.1 Mense S. Neurobiologische Mechanismen der Ubertragung von Muskelschmerz [Neurobiological mechanisms of muscle pain referral.]. Schmerz. 1993 Dec;7(4):241-9. German. doi: 10.1007/BF02529860. PMID: 18415388.
- ↑ Simmons J, Ricketson R, McMillin J. Painful lumbosacral sensory distribution patterns: Embryogenesis to adulthood. Orthopaedic review, 1993, 22(10):1110-8
- ↑ Mevlut Y. The axon reflex" Neuroanatomy: An Annual Interdisciplinary Journal of Neuroanatomy. 2008.Print.
- ↑ Lidbeck J. Central hyperexcitability in chronic musculoskeletal pain: a conceptual breakthrough with multiple clinical implications. Pain Res Manag. 2002 Summer;7(2):81-92. doi: 10.1155/2002/310974. PMID: 12185372.
- ↑ 11.0 11.1 11.2 Da Silva J.A. Pereira, Woolf Anthony D. (eds.) Rheumatology in Practice. Springer, 2010. — 533 p. — ISBN: 978-1-84882-580-2
- ↑ 12.0 12.1 12.2 12.3 12.4 Mehta N, George E. Head, Face, and Neck Pain Science, Evaluation, and Management ; John Wiley & Sons, 20-Sept-2011, p. 534.
- ↑ 13.0 13.1 Graven-Nielsen T. et al, Stimulus-response functions in areas with experimentally induced referred muscle pain- a psychophysical study, Brain Res, 1997, 744,p. 121-8.
- ↑ Graven-Nielsen T. et al, Quantification of local and referred muscle pain in humans after sequential i.m. injections of hypertonic saline, Pain 1997,69, p. 111-7.