Pain-Modulation
Pain Modulation[edit | edit source]
Pain modulation is the process of alterations in the pain signals along the transmission pathway of pain.
It explains:
- Why individuals respond to the same stimulus differently.
- The mechanism of action when using clinical analgesia.
Pain control and modulation is a complex chore that is often the primary reason patients seek the services of rehabilitation professionals.
Modulation of pain begins with an understanding of the various levels of pain modulation and extends to clinical interventions and protocols designed to reduce pain. For example, opiates are capable of increasing and decreasing pain experience.
Levels of Pain Modulation[edit | edit source]
Pain modulation is easily classified into five levels of interaction.These levels correspond to either: important synaptic junctions or significant chemical processes involved in the transmission of pain.
Level 1: Periphery[edit | edit source]
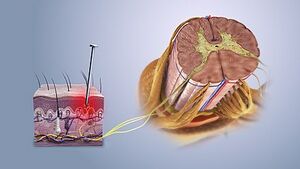
Level 1 pain modulation refers to events acting in the periphery of the body, at the site of the pain source. The somatosensation defined as the sensation from the skin, mucus, limbs, and joints and classified into: thermoception, nociception, equilibrioception mechanoreception response to (vibration, touch, and pressure), and proprioception.
Nociceptors are peripheral cell nerve endings that initiate pain sensation, respond to a noxious stimulus (thermal, mechanical, or chemical) which in turn trigger action potential to the spinal cord and to higher centers.
It is divided into A-delta and C fibers:
- A-delta fibers are large(larger than C- fiber) myelinated, fast conducting fibers, concerned with localized, sharp, and fast sensation of pain.
- C- fibers are small, unmyelinated, slow conducting nerve fibers, concerned with dull, and slow pain sensation.
Under normal conditions, the nociceptors are inactive when there is a noxious stimulus (tissue damage) those pain receptors respond according to the stimulus type and cyclooxygenase-2 is activated to release more PG(prostaglandin) at the site of injury, and the nociceptors will transmit signals to the dorsal horn of spinal cord (first-order neuron) where the first neuron release chemical substance P to transmit signals..
A- beta are myelinated, large diameter, and have the fastest conduction velocity. These fibers respond to non-painful stimuli such as:
- Touch sensation.
- Mild pressure.
- Vibration.
A beta stimulated by deep touch that explains why rubbing the painful site reliefs your pain[1][2].
Level 2: Dorsal Horn[edit | edit source]
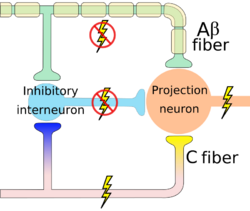
Level 2 pain modulation refers to events in the dorsal horn of the spinal cord. The modulation at the level of the spinal cord takes place at Substantial Gelatenosa of Ronaldo SG under the effect of gate control theory (GCT) which was the first theory to propose that pain experience is not only the outcome of a linear process that begins with pain pathway stimulation in the peripheral nervous system and ends with pain experience in the central nervous system. Rather, neural impulses from the peripheral nervous system that could represent pain are modulated in the spinal cord by a "gatelike" process in the dorsal horn before being transferred to the central nervous system. [3]
When small nociceptors fibers are stimulated by noxious stimulus, action potential signals are transmitted which in turn inhibit the inhibitory interneuron (I) and the projection neuron is activated so the gate is opened and pain signals are transmitted to the brain. When action potential transmits through the first-order neuron that in turn activates the vesicles to release the substance P which propagates the transmission of pain signals.
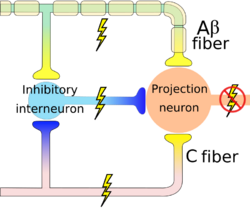
If large sensory fibers(A-Beta) are activated by deep touch for example, the transmitted signals activate the inhibitory interneuron and which blocks the projection neuron, the gate is close and there is no pain[4]. Interruption of pain signals at dorsal horn has an effect on spinothalamic tract to the cortex.
The modulation at the level of the spinal cord produces a localized analgesic effect and it receives another control from the descending pathway to cause diffuse inhibition of pain.
Level 3: Fast Neuronal Descending Pathways and Endogenous[edit | edit source]
The descending inhibitory pathway depends on the release of opioids at the SG which controls or inhibits signals transmission between the the 1st and 2nd order neuron. The descending pathway starts from periaqueductal gray matter (PAG) in the midbrain, then to raphe nucleus in the medulla to the dorsal horn of the spinal cord where these pathways release serotonin and noradrenergic neuron to inhibit the release of substance P from the presynaptic cleft of the first order neuron.Also stimulate the inhibitory interneuron to release opioids (endorphin, enkephalin) which in turn inhibit the presynaptic form releasing substance P and inhibit the post synapse of the second-order neuron from transmitting signals.
Level 4: Cortical[edit | edit source]
The noxious stimulus transmit to the cortex via spinothalamic tract that relay as a 3rd interneuron in the somatosensory area. Pain signals at the cortex induce pain modulation by two mechanisms:
- The cortex activates the PAG in the midbrain so it activates the descending pathway (top-down control of pain ) which interrupts and inhibits pain signals at the dorsal horn so by extension the spinothalamic tract is inhibited[6].
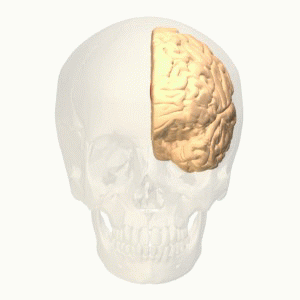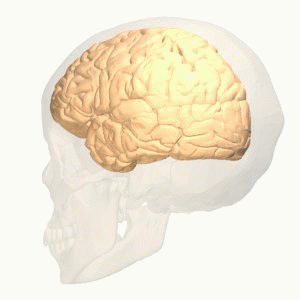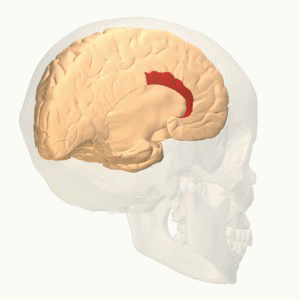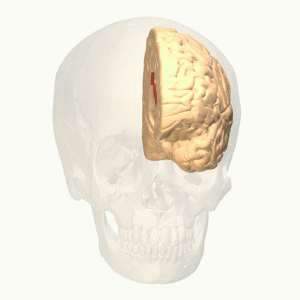
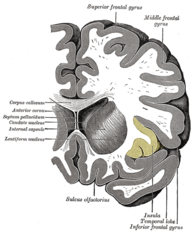
- The interaction between different areas; cerebral cortex, limbic forebrain structures, basal ganglia. The importance of this interaction is to perceive the noxious stimulus but as a non-painful. For example activation of the Anterior cingulate cortex (ACC) and the Rostral agranular insular cortex (RAIC) by noxious stimulus leads to an activation of these areas and an increase in the regional cerebral blood flow (rCBF). ACC plays a role in pain transmission. It is believed to be related to placebo analgesia and has a role with conditioned learning[7][8].
Rostral agranular insular cortex also affect on the descending inhibitory pathway and is responsible for pain learning and memory. There is a study suggest that paraventricular hypothalamic nucleus send projections of oxytocinergic that support GABA neurotransmission and activate descending spinal noradrenergic mechanisms[9].
Physical Therapy Interventions for Pain Modulation.[edit | edit source]
A systematic review delivered by Arribas-Romano A, et al 2020, demonstrated that the physiotherapy modalities can alert pain perception with chronic musculoskeletal pain CMP through:[10]
- Decrease in temporal summation
- Increase conditioned pain modulation
- Slight improvement in central sensitization
- Manual therapy.
- strengthening exercises .
A combination of more than techniques demonstrated a significant increase in conditioned pain modulation.
Manual Therapy[edit | edit source]
Manual therapy shows an increase in the activity of cortical pain modulatory regions such as the insular cortex (RAIC) and periaqueductal gray substance (PAG)[10].
- Myofascial release and Massage.
- Joint mobilization, low-velocity mobilizations[10].
- Spinal manipulative therapy: reduces the pain expectancy and strain occurs with exercise[11]
Modalities[edit | edit source]
Transcranial magnetic stimulation/ direct current stimulation.[edit | edit source]
- An important modality to reduce perception in chronic pain conditions and proved to have a significant difference when compared to a sham technique[12].
- The precentral cortical area of the motor cortex is the most target area for pain modulation.
Transcutaneous Electrical Nerve Stimulation (TENS).[edit | edit source]
TENS mechanism depend on:
- Gate control theory.
- Release of endorphins and encephalin.
Low Level Laser Therapy.[edit | edit source]
Acupuncture[edit | edit source]
- Acupuncture is used as a complementary modality for pain management, and it is mechanism depends on releasing of endogenous opioids, serotonin, and norepinephrine[13].
- The variability in its effect depends on:
- The method of application.
- The number of needles.
- The duration of application.
- There are studies with low to moderate quality that demonstrated a decrease in pain intensity with modest benefits especially with chronic pain( chronic low back pain, tension headache, chronic headache, migraine headache, and myofascial pain)[14]but slight improvement with acute conditions and this improvement is not clinically significant and may refer it to the placebo effect [13].
Dry needling.[edit | edit source]
Interferential Therapy[edit | edit source]
Exercise[edit | edit source]
For how exercises affect on pain and what are recommended exercises you can read: Exercise and Activity in Pain Management
Resources[edit | edit source]
UTHealth, the University of Texas
References[edit | edit source]
- ↑ Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, Williams SM. Nociceptors. Sunderland, MA. 2001.
- ↑ Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. International journal of molecular sciences. 2018 Aug;19(8):2164.
- ↑ Galik, E., Fukudo, S., Tanaka, Y., Gidron, Y., Campbell, T. S., Johnson, J. A., Zernicke, K. A., Pellowski, J., García, L. I., Mitchell, J. W., Erausquin, J. T., Salem, R. M., Rodriguez-Murillo, L., Chiba-Falek, O., Jiang, R., Jiang, R., Campbell, T. S., Johnson, J. A., Zernicke, K. A., … Anderson, G. M. (2013). Gate control theory of pain. In Encyclopedia of Behavioral Medicine (pp. 832–834). Springer New York.
- ↑ Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters involved in pain regulation. International journal of molecular sciences. 2018 Aug;19(8):2164.
- ↑ Armando Hasudungan. PAIN! Physiology - The Ascending Pathway, Descending Pain Pathway, and the Substantia Gelatinosa. Available from: http://www.youtube.com/watch?v=5c8maFAhqIc[last accessed 11/12/2021]
- ↑ Ohara PT, Vit JP, Jasmin L. Cortical modulation of pain. Cellular and Molecular Life Sciences CMLS. 2005 Jan;62(1):44-52.
- ↑ Xie YF, Huo FQ, Tang JS. Cerebral cortex modulation of pain. Acta Pharmacologica Sinica. 2009 Jan;30(1):31-41.
- ↑ Steeds CE. The anatomy and physiology of pain. Surgery (Oxford). 2009 Dec 1;27(12):507-11.
- ↑ Gamal-Eltrabily M, de Los Monteros-Zúñiga AE, Manzano-García A, Martínez-Lorenzana G, Condés-Lara M, González-Hernández A. The rostral agranular insular cortex, a new site of oxytocin to induce antinociception. Journal of Neuroscience. 2020 Jul 15;40(29):5669-80.
- ↑ 10.0 10.1 10.2 Arribas-Romano A, Fernández-Carnero J, Molina-Rueda F, Angulo-Diaz-Parreño S, Navarro-Santana MJ. Efficacy of physical therapy on nociceptive pain processing alterations in patients with chronic musculoskeletal pain: a systematic review and meta-analysis. Pain Medicine. 2020 Oct;21(10):2502-17.
- ↑ Ellingsen DM, Napadow V, Protsenko E, Mawla I, Kowalski MH, Swensen D, O'Dwyer-Swensen D, Edwards RR, Kettner N, Loggia ML. Brain mechanisms of anticipated painful movements and their modulation by manual therapy in chronic low back pain. The journal of pain. 2018 Nov 1;19(11):1352-65.
- ↑ Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain. 2012 Jul 1;153(7):1350-63.
- ↑ 13.0 13.1 Kelly RB, Willis J. Acupuncture for pain. American family physician. 2019 Jul 15;100(2):89-96.
- ↑ Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, Fu R, Brodt ED, Wasson N, Winter C, Ferguson AJ. Noninvasive nonpharmacological treatment for chronic pain: a systematic review.