Pain Behaviours
Original Editor - Joanne Garvey.
Top Contributors - Joanne Garvey, Kapil Narale, Jo Etherton, Aminat Abolade, Evan Thomas, Tarina van der Stockt, Kim Jackson, Lucinda hampton, Melissa Coetsee, Rachael Lowe, Lauren Lopez, Carina Therese Magtibay and Aya Alhindi
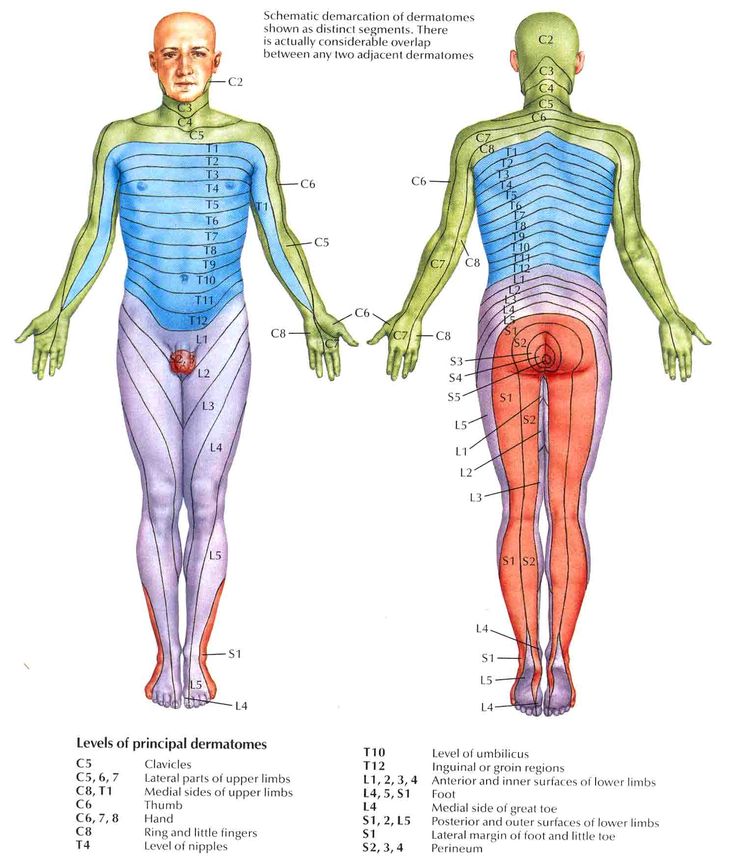
Referred Pain[edit | edit source]
Referred pain is pain perceived at a location other than the site of the painful stimulus. It usually originates in one of the visceral organs but is felt in the skin or sometimes in another area deep inside the body. Its mechanism is likely due to the fact that pain signals from the viscera travel along the same neural pathways used by pain signals from the skin. The result is the perception of pain originating in the skin rather than in a deep-seated visceral organ or neural structure.
Visceral nociceptors project into the spinal cord via small diameter myelinated and unmyelinated fibres from the autonomic nervous system. They go on to synapse close to the point of embryonic origin in the spine. When an organ develops pain, it can therefore be perceived as being at a different point in the body, on the surface, rather than in the organ itself.[1]
Mechanism
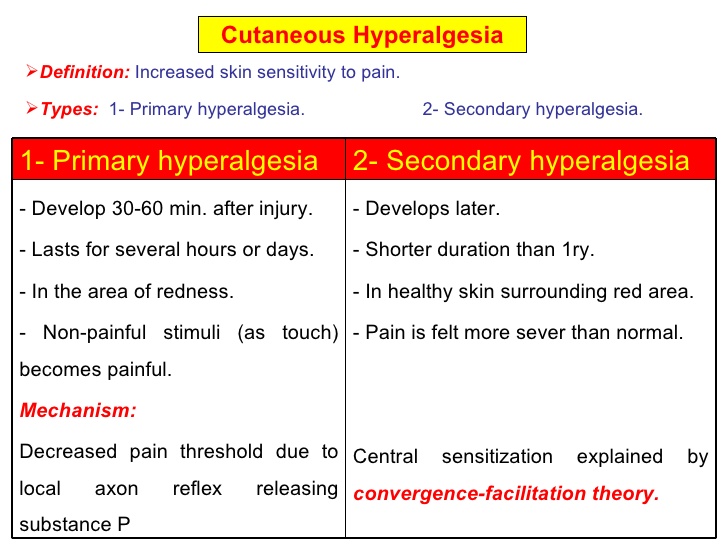
Hyperalgesia
[edit | edit source]
Hyperalgesia is defined as "An increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves".[2]
It is divided into 2 types, primary and secondary.
- Primary hyperalgesia - pain and sensitivity in the damaged tissues.
- Secondary hyperalgesia - pain and sensitivity that occurs in area around the damaged tissues.
Substance P appears to have a significant role in the sensitiation of nociceptors, which may explain the heightened feeling of pain in injured tissue (primary hyperalgesia). Although this doesn't account for the perception of non-painful stimuli as painful. Secondary hyperalgesia is most likely accounted for by changes in the dorsal horn which affect the processing of sensory information. [1] It is likely that normally silent nociceptors are recruited, and sprouting of large diameter sensory afferent occurs projecting into the dorsal horn laminae.
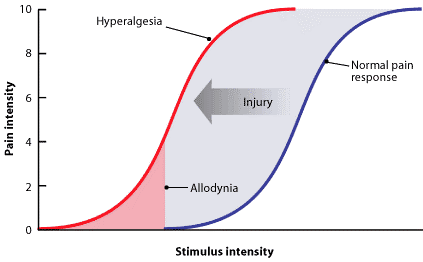
Allodynia[edit | edit source]
Central pain sensitization (increased response of neurons) following painful, often repetitive, stimulation. Allodynia can lead to the triggering of a pain response from stimuli which do not normally provoke pain.[3]
Types of allodynia:
- Mechanical allodynia (also known as tactile allodynia)
- Static mechanical allodynia – pain in response to light touch/pressure[4]
- Dynamic mechanical allodynia – pain in response to stroking lightly[5]
- Thermal (hot or cold) allodynia – pain from normally mild skin temperatures in the affected area
- Movement allodynia - pain triggered by normal movement of joints or muscles.
Pathophysiology
Cellular level
The cell types involved in nociception and mechanical sensation are the cells responsible for allodynia. In healthy individuals, nociceptors sense information about cell stress or damage and temperature at the skin and transmit it to the spinal cord. The cell bodies of these neurons lie in dorsal root ganglia, important structures located on both sides of the spinal cord. The axons then pass through the dorsal horn to make connections with secondary neurons. The secondary neurons cross over to the other (contralateral) side of the spinal cord and reach nuclei of the thalamus. From there, the information is carried through one or more neurons to the somatosensory cortex of the brain. Mechanoreceptors follow the same general pathway. However, they do not cross over at the level of the spinal cord, but at the lower medulla instead. In addition, they are grouped in tracts that are spatially distinct from the nociceptive tracts.
Despite this anatomical separation, mechanoreceptors can influence the output of nociceptors by making connections with the same interneurons, the activation of which can reduce or completely eliminate the sensation of pain. Another way to modulate the transmission of pain information is via descending fibers from the brain. These fibers act through different interneurons to block the transmission of information from the nociceptors to secondary neurons.[6]
Both of these mechanisms for pain modulation have been implicated in the pathology of allodynia. Several studies suggest that injury to the spinal cord might lead to loss and re-organization of the nociceptors, mechanoreceptors and interneurons, leading to the transmission of pain information by mechanoreceptors[7][8]A different study reports the appearance of descending fibers at the injury site.[9] All of these changes ultimately affect the circuitry inside the spinal cord, and the altered balance of signals probably leads to the intense sensation of pain associated with allodynia.
Different cell types have also been linked to allodynia. For example, there are reports that microglia in the thalamus might contribute to allodynia by changing the properties of the secondary nociceptors.[10] The same effect is achieved in the spinal cord by the recruitment of immune system cells such as monocytes/macrophages and T lymphocytes.[11]
Molecular level
There is a strong body of evidence that the so-called sensitization of the central nervous system contributes to the appearance of allodynia. Sensitization refers to the increased response of neurons following repetitive stimulation. In addition to repeated activity, the increased levels of certain compounds lead to sensitization, as well. The work of many researchers has led to the elucidation of pathways that can result in neuronal sensitization both in the thalamus and dorsal horns. Both pathways depend on the production of chemokines and other molecules important in the inflammatory response.
A very important molecule in the thalamus appears to be cysteine-cysteine chemokine ligand 21 (CCL21). The concentration of this chemokine is increased in the ventral posterolateral nucleus of the thalamus where secondary nociceptive neurons make connections with other neurons. The source of CCL21 is not exactly known, but two possibilities exist. First, it might be made in primary nociceptive neurons and transported up to the thalamus. Most likely, neurons intrinsic to the ventral posterolateral nucleus make at least some of it. In any case, CCL21 binds to C-C chemokine receptor type 7 and chemokine receptor CXCR3 receptors on microglia in the thalamus.[12]The physiologic response to the binding is probably the production of prostaglandin E2 (PGE2) by cyclooxygenase 2 (COX-2).[13]Activated microglia making PGE2 can then sensitize nociceptive neurons as manifested by their lowered threshold to pain.[14]
The mechanism responsible for sensitization of the central nervous system at the level of the spinal cord is different from the one in the thalamus. Tumor necrosis factor-alpha (TNF-alpha) and its receptor are the molecules that seem to be responsible for the sensitization of neurons in the dorsal horns of the spinal cord. Macrophages and lymphocytes infiltrate the spinal cord, for example, because of injury, and release TNF-alpha and other pro-inflammatory molecules.[15]TNF-alpha then binds to the TNF receptors expressed on nociceptors, activating the MAPK/NF-kappa B pathways. This leads to the production of more TNF-alpha, its release, and binding to the receptors on the cells that released it (autocrine signalling). This mechanism also explains the perpetuation of sensitization and thus allodynia. TNF-alpha might also increase the number of AMPA receptors, and decrease the numbers of GABA receptors on the membrane of nociceptors, both of which could change the nociceptors in a way that allows for their easier activation.[16] Another outcome of the increased TNF-alpha is the release of PGE2, with a mechanism and effect similar to the ones in the thalamus.[17].
Inflammatory Pain[edit | edit source]
One of the cardinal features of inflammatory states is that normally innocuous stimuli produce pain[18]
Please follow the link to this article for an overview of Inflammatory pain
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ 1.0 1.1 Roger, Barker, Barasi, Neal. Neuroscience at a glance.1999 Blackwell science.
- ↑ Hart BL (1988). "Biological basis of the behavior of sick animals". Neurosci Biobehav Rev 12 (2): 123–37.
- ↑ Merskey & Bogduk (Eds.) Classification of Chronic Pain. Seattle: IASP Task Force on Taxonomy, 1994
- ↑ Attal N, Brasseur L, Chauvin M, Bouhassira D (1999). "Effects of single and repeated applications of a eutectic mixture of local anaesthetics (EMLA) cream on spontaneous and evoked pain in post-herpetic neuralgia". Pain 81 (1–2): 203–9
- ↑ LoPinto C, Young WB, Ashkenazi A (2006). "Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine". Cephalalgia 26 (7): 852–6.
- ↑ Fitzpatrick, David; Purves, Dale; Augustine, George (2004). Neuroscience. Sunderland, Mass: Sinauer. pp. 231–250.
- ↑ Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL (1998). "Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model". Pain 75 (1): 141–55
- ↑ Wasner G, Naleschinski D, Baron R (2007). "A role for peripheral afferents in the pathophysiology and treatment of at-level neuropathic pain in spinal cord injury? A case report". Pain 131 (1–2): 219–25
- ↑ Kalous A, Osborne PB, Keast JR (2007). "Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury". J. Comp. Neurol. 504 (3): 238–53
- ↑ Zhao P, Waxman SG, Hains BC (2007). "Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21". J. Neurosci. 27 (33): 8893–902.
- ↑ Wei XH, Zang Y, Wu CY, Xu JT, Xin WJ, Liu XG (2007). "Peri-sciatic administration of recombinant rat TNF-alpha induces mechanical allodynia via upregulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: the role of NF-kappa B pathway". Exp. Neurol. 205 (2): 471–84.
- ↑ Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K (2006). "Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo". Glia 54 (8): 861–72
- ↑ Alique M, Herrero JF, Lucio-Cazana FJ (2007). "All-trans retinoic acid induces COX-2 and prostaglandin E2 synthesis in SH-SY5Y human neuroblastoma cells: involvement of retinoic acid receptors and extracellular-regulated kinase 1/2". J Neuroinflammation 4: 1
- ↑ Rukwied R, Chizh BA, Lorenz U (2007). "Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2". J Pain 8 (5): 443–51.
- ↑ Haskó G, Pacher P, Deitch EA, Vizi ES (2007). "Shaping of monocyte and macrophage function by adenosine receptors". Pharmacol. Ther. 113 (2): 264–75.
- ↑ Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005). "Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha". J. Neurosci. 25 (12): 3219–28.
- ↑ Coutaux A, Adam F, Willer JC, Le Bars D (2005). "Hyperalgesia and allodynia: peripheral mechanisms". Joint Bone Spine 72 (5): 359–71.
- ↑ B. L. Kidd1 and L. A. Urban2. Mechanisms of inflammatory pain. Oxford JournalsMedicine & Health BJA Volume 87, Issue 1Pp. 3-11