Pain Behaviours: Difference between revisions
No edit summary |
No edit summary |
||
| Line 23: | Line 23: | ||
'''Mechanism''' | '''Mechanism''' | ||
There are several proposed mechanisms for referred pain. Currently there is no definitive consensus regarding which is correct. The cardiac general visceral sensory pain fibers follow the sympathetics back to the spinal cord and have their cell bodies located in thoracic dorsal root ganglia 1-4<ref name="simons">Simons, D.G.; Travell, J.G.; Simons, L.S. (1999). Travell &amp;amp;amp;amp;amp;amp;amp; Simons' Myofascial Pain and Dysfunction: Upper half of body. Williams &amp;amp;amp;amp;amp;amp;amp; Wilkins. p. 750</ref>. As a general rule, in the thorax and abdomen, general visceral afferent (GVA) pain fibers follow sympathetic fibers back to the same spinal cord segments that gave rise to the preganglionic sympathetic fibers. The central nervous system (CNS) perceives pain from the heart as coming from the somatic portion of the body supplied by the thoracic spinal cord segments 1-4<ref name="simons" />. Classically the pain associated with a myocardial infarction is located in the mid or left side of the chest where the heart is actually located. The pain can radiate to the left side of the jaw and into the left arm. Myocardial infarction can rarely present as referred pain and this usually occurs in people with<ref name="myocardial">"Myocardial infarction comes with referred pain or radiating pain?". January 2, 2011. Retrieved December 26, 2011</ref> diabetes or older age. Also, the dermatomes of this region of the body wall and upper limb have their neuronal cell bodies in the same dorsal root ganglia (T1-5) and synapse in the same second order neurons in the spinal cord segments (T1-5) as the general visceral sensory fibers from the heart. The CNS does not clearly discern whether the pain is coming from the body wall or from the viscera, but it perceives the pain as coming from somewhere on the body wall, i.e. substernal pain, left arm/hand pain, jaw pain. | There are several proposed mechanisms for referred pain. Currently there is no definitive consensus regarding which is correct. The cardiac general visceral sensory pain fibers follow the sympathetics back to the spinal cord and have their cell bodies located in thoracic dorsal root ganglia 1-4<ref name="simons">Simons, D.G.; Travell, J.G.; Simons, L.S. (1999). Travell &amp;amp;amp;amp;amp;amp;amp;amp; Simons' Myofascial Pain and Dysfunction: Upper half of body. Williams &amp;amp;amp;amp;amp;amp;amp;amp; Wilkins. p. 750</ref>. As a general rule, in the thorax and abdomen, general visceral afferent (GVA) pain fibers follow sympathetic fibers back to the same spinal cord segments that gave rise to the preganglionic sympathetic fibers. The central nervous system (CNS) perceives pain from the heart as coming from the somatic portion of the body supplied by the thoracic spinal cord segments 1-4<ref name="simons" />. Classically the pain associated with a myocardial infarction is located in the mid or left side of the chest where the heart is actually located. The pain can radiate to the left side of the jaw and into the left arm. Myocardial infarction can rarely present as referred pain and this usually occurs in people with<ref name="myocardial">"Myocardial infarction comes with referred pain or radiating pain?". January 2, 2011. Retrieved December 26, 2011</ref> diabetes or older age. Also, the dermatomes of this region of the body wall and upper limb have their neuronal cell bodies in the same dorsal root ganglia (T1-5) and synapse in the same second order neurons in the spinal cord segments (T1-5) as the general visceral sensory fibers from the heart. The CNS does not clearly discern whether the pain is coming from the body wall or from the viscera, but it perceives the pain as coming from somewhere on the body wall, i.e. substernal pain, left arm/hand pain, jaw pain. | ||
'''Convergent Projection''' | '''Convergent Projection''' | ||
| Line 51: | Line 51: | ||
Thalamic convergence suggests that referred pain is perceived as such due to the summation of neural inputs in the brain, as opposed to the spinal cord, from the injured area and the referred area. Experimental evidence on thalamic convergence is lacking. However, pain studies performed on monkeys revealed several.<br> | Thalamic convergence suggests that referred pain is perceived as such due to the summation of neural inputs in the brain, as opposed to the spinal cord, from the injured area and the referred area. Experimental evidence on thalamic convergence is lacking. However, pain studies performed on monkeys revealed several.<br> | ||
<br> {{#ev:youtube|wbpjEPRq6pA}} | |||
<br> <br> | |||
[[Image:Dermatome.jpg|frame|center|Dermatomal distribution of referred pain]] | |||
[[Image:Dermatome.jpg|frame|center|Dermatomal distribution of referred pain]] | |||
== Hyperalgesia<br> == | == Hyperalgesia<br> == | ||
| Line 84: | Line 82: | ||
The release of proinflammatory cytokines such as Interleukin-1 by activated leukocytes triggered by lipopolysaccharides, endotoxins and other signals of infection also increases pain sensitivity as part of sickness behavior, the evolved response to illness.<ref name="maier">Maier SF, Wiertelak EP, Martin D, Watkins LR (October 1993). "Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin". Brain Res. 623 (2): 321–4.</ref> | The release of proinflammatory cytokines such as Interleukin-1 by activated leukocytes triggered by lipopolysaccharides, endotoxins and other signals of infection also increases pain sensitivity as part of sickness behavior, the evolved response to illness.<ref name="maier">Maier SF, Wiertelak EP, Martin D, Watkins LR (October 1993). "Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin". Brain Res. 623 (2): 321–4.</ref> | ||
<br> | |||
[[Image:Hyperalgesia.jpg|frame|center|Primary and Secondary hyperalgesia Comparison]] | |||
[[Image:Hyperalgesia.jpg|frame|center|Primary and Secondary hyperalgesia Comparison]] | |||
== Allodynia == | == Allodynia == | ||
Central pain sensitization (increased response of neurons) following painful, often repetitive, stimulation. Allodynia can lead to the triggering of a pain response from stimuli which do not normally provoke pain.<ref name="merskey">Merskey &amp;amp; Bogduk (Eds.) Classification of Chronic Pain. Seattle: IASP Task Force on Taxonomy, 1994</ref><br> | Central pain sensitization (increased response of neurons) following painful, often repetitive, stimulation. Allodynia can lead to the triggering of a pain response from stimuli which do not normally provoke pain.<ref name="merskey">Merskey &amp;amp;amp; Bogduk (Eds.) Classification of Chronic Pain. Seattle: IASP Task Force on Taxonomy, 1994</ref><br> | ||
<br> | <br> | ||
| Line 118: | Line 116: | ||
A very important molecule in the thalamus appears to be cysteine-cysteine chemokine ligand 21 (CCL21). The concentration of this chemokine is increased in the ventral posterolateral nucleus of the thalamus where secondary nociceptive neurons make connections with other neurons. The source of CCL21 is not exactly known, but two possibilities exist. First, it might be made in primary nociceptive neurons and transported up to the thalamus. Most likely, neurons intrinsic to the ventral posterolateral nucleus make at least some of it. In any case, CCL21 binds to C-C chemokine receptor type 7 and chemokine receptor CXCR3 receptors on microglia in the thalamus.<ref>Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K (2006). "Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo". Glia 54 (8): 861–72</ref>The physiologic response to the binding is probably the production of prostaglandin E2 (PGE2) by cyclooxygenase 2 (COX-2).<ref>Alique M, Herrero JF, Lucio-Cazana FJ (2007). "All-trans retinoic acid induces COX-2 and prostaglandin E2 synthesis in SH-SY5Y human neuroblastoma cells: involvement of retinoic acid receptors and extracellular-regulated kinase 1/2". J Neuroinflammation 4: 1</ref>Activated microglia making PGE2 can then sensitize nociceptive neurons as manifested by their lowered threshold to pain.<ref>Rukwied R, Chizh BA, Lorenz U (2007). "Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2". J Pain 8 (5): 443–51.</ref> | A very important molecule in the thalamus appears to be cysteine-cysteine chemokine ligand 21 (CCL21). The concentration of this chemokine is increased in the ventral posterolateral nucleus of the thalamus where secondary nociceptive neurons make connections with other neurons. The source of CCL21 is not exactly known, but two possibilities exist. First, it might be made in primary nociceptive neurons and transported up to the thalamus. Most likely, neurons intrinsic to the ventral posterolateral nucleus make at least some of it. In any case, CCL21 binds to C-C chemokine receptor type 7 and chemokine receptor CXCR3 receptors on microglia in the thalamus.<ref>Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K (2006). "Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo". Glia 54 (8): 861–72</ref>The physiologic response to the binding is probably the production of prostaglandin E2 (PGE2) by cyclooxygenase 2 (COX-2).<ref>Alique M, Herrero JF, Lucio-Cazana FJ (2007). "All-trans retinoic acid induces COX-2 and prostaglandin E2 synthesis in SH-SY5Y human neuroblastoma cells: involvement of retinoic acid receptors and extracellular-regulated kinase 1/2". J Neuroinflammation 4: 1</ref>Activated microglia making PGE2 can then sensitize nociceptive neurons as manifested by their lowered threshold to pain.<ref>Rukwied R, Chizh BA, Lorenz U (2007). "Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2". J Pain 8 (5): 443–51.</ref> | ||
The mechanism responsible for sensitization of the central nervous system at the level of the spinal cord is different from the one in the thalamus. Tumor necrosis factor-alpha (TNF-alpha) and its receptor are the molecules that seem to be responsible for the sensitization of neurons in the dorsal horns of the spinal cord. Macrophages and lymphocytes infiltrate the spinal cord, for example, because of injury, and release TNF-alpha and other pro-inflammatory molecules.<ref>Haskó G, Pacher P, Deitch EA, Vizi ES (2007). "Shaping of monocyte and macrophage function by adenosine receptors". Pharmacol. Ther. 113 (2): 264–75.</ref>TNF-alpha then binds to the TNF receptors expressed on nociceptors, activating the MAPK/NF-kappa B pathways. This leads to the production of more TNF-alpha, its release, and binding to the receptors on the cells that released it (autocrine signalling). This mechanism also explains the perpetuation of sensitization and thus allodynia. TNF-alpha might also increase the number of AMPA receptors, and decrease the numbers of GABA receptors on the membrane of nociceptors, both of which could change the nociceptors in a way that allows for their easier activation.<ref>Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005). "Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha". J. Neurosci. 25 (12): 3219–28.</ref> Another outcome of the increased TNF-alpha is the release of PGE2, with a mechanism and effect similar to the ones in the thalamus.<ref>Coutaux A, Adam F, Willer JC, Le Bars D (2005). "Hyperalgesia and allodynia: peripheral mechanisms". Joint Bone Spine 72 (5): 359–71.</ref>. | The mechanism responsible for sensitization of the central nervous system at the level of the spinal cord is different from the one in the thalamus. Tumor necrosis factor-alpha (TNF-alpha) and its receptor are the molecules that seem to be responsible for the sensitization of neurons in the dorsal horns of the spinal cord. Macrophages and lymphocytes infiltrate the spinal cord, for example, because of injury, and release TNF-alpha and other pro-inflammatory molecules.<ref>Haskó G, Pacher P, Deitch EA, Vizi ES (2007). "Shaping of monocyte and macrophage function by adenosine receptors". Pharmacol. Ther. 113 (2): 264–75.</ref>TNF-alpha then binds to the TNF receptors expressed on nociceptors, activating the MAPK/NF-kappa B pathways. This leads to the production of more TNF-alpha, its release, and binding to the receptors on the cells that released it (autocrine signalling). This mechanism also explains the perpetuation of sensitization and thus allodynia. TNF-alpha might also increase the number of AMPA receptors, and decrease the numbers of GABA receptors on the membrane of nociceptors, both of which could change the nociceptors in a way that allows for their easier activation.<ref>Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005). "Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha". J. Neurosci. 25 (12): 3219–28.</ref> Another outcome of the increased TNF-alpha is the release of PGE2, with a mechanism and effect similar to the ones in the thalamus.<ref>Coutaux A, Adam F, Willer JC, Le Bars D (2005). "Hyperalgesia and allodynia: peripheral mechanisms". Joint Bone Spine 72 (5): 359–71.</ref>. | ||
<br> | |||
[[Image:Allodynia.gif|frame|center]] | |||
== Inflammatory Pain == | |||
== References == | == References == | ||
Revision as of 17:10, 1 April 2016
- Please do not edit unless you are involved in this project, but please come back in the near future to check out new information!!
- If you would like to get involved in this project and earn accreditation for your contributions, please get in touch!
Tips for writing this page:
- Define the following and describe the underlying mechanisms: referred pain, primary hyperalgesia, secondary hyperalgesia, allodynia etc
Original Editor - Add a link to your Physiopedia profile here.
Top Contributors - Joanne Garvey, Kapil Narale, Aminat Abolade, Jo Etherton, Kim Jackson, Lucinda hampton, Melissa Coetsee, Evan Thomas, Tarina van der Stockt, Lauren Lopez, Carina Therese Magtibay, Aya Alhindi and Rachael Lowe
Referred Pain[edit | edit source]
Referred pain, is pain perceived at a location other than the site of the painful stimulus.
Referred pain is pain in a part other than that in which the cause that produced it is situated. Referred pain usually originates in one of the visceral organs but is felt in the skin or sometimes in another area deep inside the body. It probably occurs because pain signals from the viscera travel along the same neural pathways used by pain signals from the skin. The person perceives the pain but interprets it as having originated in the skin rather than in a deep-seated visceral organ or neural structure.
An example is the case of angina pectoris brought on by a myocardial infarction (heart attack), where pain is often felt in the neck, shoulders, and back rather than in the thorax (chest), the site of the injury. The International Association for the Study of Pain has not officially defined the term; hence several authors have defined the term differently.
Radiating pain is different from referred pain; for example, the pain related to a myocardial infarction could either be referred or radiating pain from the chest. Referred pain is when the pain is located away from or adjacent to the organ involved; for instance, when a person has pain only in their jaw or left arm, but not in the chest. Physicians and scientists have known about referred pain since the late 1880s. Despite an increasing amount of literature on the subject, the biological mechanism of referred pain is unknown, although there are several hypotheses.[1]
Mechanism
There are several proposed mechanisms for referred pain. Currently there is no definitive consensus regarding which is correct. The cardiac general visceral sensory pain fibers follow the sympathetics back to the spinal cord and have their cell bodies located in thoracic dorsal root ganglia 1-4[2]. As a general rule, in the thorax and abdomen, general visceral afferent (GVA) pain fibers follow sympathetic fibers back to the same spinal cord segments that gave rise to the preganglionic sympathetic fibers. The central nervous system (CNS) perceives pain from the heart as coming from the somatic portion of the body supplied by the thoracic spinal cord segments 1-4[2]. Classically the pain associated with a myocardial infarction is located in the mid or left side of the chest where the heart is actually located. The pain can radiate to the left side of the jaw and into the left arm. Myocardial infarction can rarely present as referred pain and this usually occurs in people with[3] diabetes or older age. Also, the dermatomes of this region of the body wall and upper limb have their neuronal cell bodies in the same dorsal root ganglia (T1-5) and synapse in the same second order neurons in the spinal cord segments (T1-5) as the general visceral sensory fibers from the heart. The CNS does not clearly discern whether the pain is coming from the body wall or from the viscera, but it perceives the pain as coming from somewhere on the body wall, i.e. substernal pain, left arm/hand pain, jaw pain.
Convergent Projection
This represents one of the earliest theories on the subject of referred pain. It is based on the work of W.A. Sturge and J. Ross from 1888 and later TC Ruch in 1961. Convergent projection proposes that afferent nerve fibers from tissues converge onto the same spinal neuron, and explains why referred pain is believed to be segmented in much the same way as the spinal cord. Additionally, experimental evidence shows that when local pain (pain at the site of stimulation) is intensified the referred pain is intensified as well.
Criticism of this model arises from its inability to explain why there is a delay between the onset of referred pain after local pain stimulation. Experimental evidence also shows that referred pain is often unidirectional. For example, stimulated local pain in the anterior tibial muscle causes referred pain in the ventral portion of the ankle; however referred pain moving in the opposite direction has not been shown experimentally. Lastly, the threshold for the local pain stimulation and the referred pain stimulation are different, but according to this model they should both be the same.[1]
Convergence Facilitation
Convergence facilitation was conceived in 1893 by J MacKenzie based on the ideas of Sturge and Ross. He believed that the internal organs were insensitive to stimuli. Furthermore, he believed that non-nociceptive afferent inputs to the spinal cord created what he termed "an irritable focus". This focus caused some stimuli to be perceived as referred pain. However, his ideas did not gain widespread acceptance from critics due to its dismissal of visceral pain.
Recently this idea has regained some credibility under a new term, central sensitization. Central sensitization occurs when neurons in the spinal cord's dorsal horn or brainstem become more responsive after repeated stimulation by peripheral neurons, so that weaker signals can trigger them. The delay in appearance of referred pain shown in laboratory experiments can be explained due to the time required to create the central sensitization.
Axon -Reflex
Axon reflex suggests that the afferent fiber is bifurcated before connecting to the dorsal horn. Bifurcated fibers do exist in muscle, skin, and intervertebral discs. Yet these particular neurons are rare and are not representative of the whole body. Axon-Reflex also does not explain the time delay before the appearance of referred pain, threshold differences for stimulating local and referred pain, and somatosensory sensibility changes in the area of referred pain.
Hyper-Excitability
Hyperexcitability hypothesizes that referred pain has no central mechanism. However, it does say that there is one central characteristic that predominates. Experiments involving noxious stimuli and recordings from the dorsal horn of animals revealed that referred pain sensations began minutes after muscle stimulation. Pain was felt in a receptive field that was some distance away from the original receptive field. According to hyperexcitability, new receptive fields are created as a result of the opening of latent convergent afferent fibers in the dorsal horn. This signal could then be perceived as referred pain.
Several characteristics are in line with this mechanism of referred pain, such as dependency on stimulus and the time delay in the appearance of referred pain as compared to local pain. However, the appearance of new receptive fields, which is interpreted to be referred pain, conflicts with the majority of experimental evidence from studies including studies of healthy individuals. Furthermore, referred pain generally appears within seconds in humans as opposed to minutes in animal models. Some scientists attribute this to a mechanism or influence downstream in the supraspinal pathways. Neuroimaging techniques such as PET scans or fMRI may visualize the underlying neural processing pathways responsible in future testing.
Thalamic-convergence
Thalamic convergence suggests that referred pain is perceived as such due to the summation of neural inputs in the brain, as opposed to the spinal cord, from the injured area and the referred area. Experimental evidence on thalamic convergence is lacking. However, pain studies performed on monkeys revealed several.
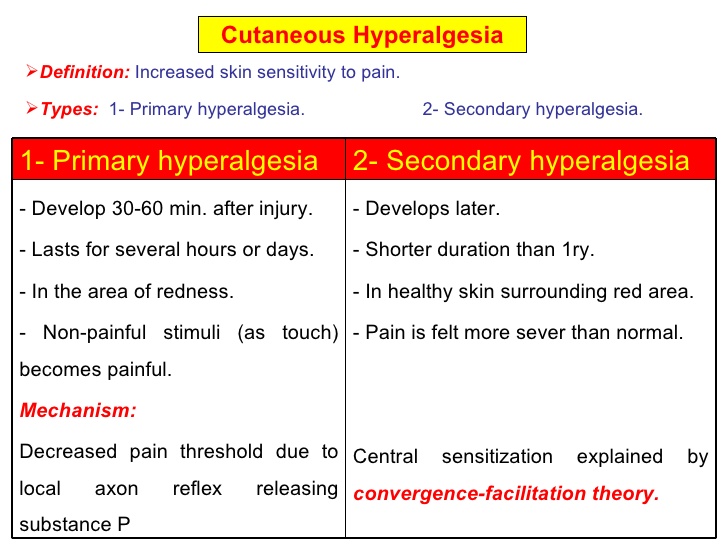
Hyperalgesia
[edit | edit source]
An increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves.[4]
Hyperalgesia can be experienced in focal, discrete areas, or as a more diffuse, body-wide form.
The focal form is typically associated with injury, and is divided into two subtypes:
- Primary hyperalgesia - pain sensitivity that occurs directly in the damaged tissues.
- Secondary hyperalgesia - pain sensitivity that occurs in surrounding undamaged tissues.
Causes
Hyperalgesia is induced by platelet-activating factor (PAF) which comes about in an inflammatory or an allergic response. This seems to occur via immune cells interacting with the peripheral nervous system and releasing pain-producing chemicals (cytokines and chemokines)[5]
Long-term opioid (e.g. heroin, morphine) users and those on high-dose opioid medications for the treatment of chronic pain, may experience hyperalgesia and experience pain out of proportion to physical findings, which is a common cause for loss of efficacy of these medications over time.[6][7] As it can be difficult to distinguish from tolerance, opioid-induced hyperalgesia is often compensated for by escalating the dose of opioid, potentially worsening the problem by further increasing sensitivity to pain. Chronic hyperstimulation of opioid receptors results in altered homeostasis of pain signalling pathways in the body with several mechanisms of action involved. One major pathway being through stimulation of the nociceptin receptor,[8] [9]and blocking this receptor may therefore be a means of preventing the development of hyperalgesia.[10]
Stimulation of nociceptive fibers in a pattern consistent with that from inflammation switches on a form of amplification in the spinal cord, long term potentiation.[11] This occurs where the pain fibres synapse to pain pathway, the periaqueductal grey. Amplification in the spinal cord may be another way of producing hyperalgesia.
The release of proinflammatory cytokines such as Interleukin-1 by activated leukocytes triggered by lipopolysaccharides, endotoxins and other signals of infection also increases pain sensitivity as part of sickness behavior, the evolved response to illness.[12]
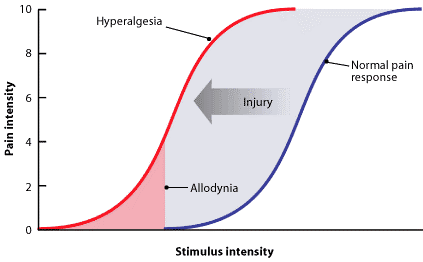
Allodynia[edit | edit source]
Central pain sensitization (increased response of neurons) following painful, often repetitive, stimulation. Allodynia can lead to the triggering of a pain response from stimuli which do not normally provoke pain.[13]
Types of allodynia:
- Mechanical allodynia (also known as tactile allodynia)
- Static mechanical allodynia – pain in response to light touch/pressure[14]
- Dynamic mechanical allodynia – pain in response to stroking lightly[15]
- Thermal (hot or cold) allodynia – pain from normally mild skin temperatures in the affected area
- Movement allodynia - pain triggered by normal movement of joints or muscles.
Pathophysiology
Cellular level
The cell types involved in nociception and mechanical sensation are the cells responsible for allodynia. In healthy individuals, nociceptors sense information about cell stress or damage and temperature at the skin and transmit it to the spinal cord. The cell bodies of these neurons lie in dorsal root ganglia, important structures located on both sides of the spinal cord. The axons then pass through the dorsal horn to make connections with secondary neurons. The secondary neurons cross over to the other (contralateral) side of the spinal cord and reach nuclei of the thalamus. From there, the information is carried through one or more neurons to the somatosensory cortex of the brain. Mechanoreceptors follow the same general pathway. However, they do not cross over at the level of the spinal cord, but at the lower medulla instead. In addition, they are grouped in tracts that are spatially distinct from the nociceptive tracts.
Despite this anatomical separation, mechanoreceptors can influence the output of nociceptors by making connections with the same interneurons, the activation of which can reduce or completely eliminate the sensation of pain. Another way to modulate the transmission of pain information is via descending fibers from the brain. These fibers act through different interneurons to block the transmission of information from the nociceptors to secondary neurons.[16]
Both of these mechanisms for pain modulation have been implicated in the pathology of allodynia. Several studies suggest that injury to the spinal cord might lead to loss and re-organization of the nociceptors, mechanoreceptors and interneurons, leading to the transmission of pain information by mechanoreceptors[17][18]A different study reports the appearance of descending fibers at the injury site.[19] All of these changes ultimately affect the circuitry inside the spinal cord, and the altered balance of signals probably leads to the intense sensation of pain associated with allodynia.
Different cell types have also been linked to allodynia. For example, there are reports that microglia in the thalamus might contribute to allodynia by changing the properties of the secondary nociceptors.[20] The same effect is achieved in the spinal cord by the recruitment of immune system cells such as monocytes/macrophages and T lymphocytes.[21]
Molecular level
There is a strong body of evidence that the so-called sensitization of the central nervous system contributes to the appearance of allodynia. Sensitization refers to the increased response of neurons following repetitive stimulation. In addition to repeated activity, the increased levels of certain compounds lead to sensitization, as well. The work of many researchers has led to the elucidation of pathways that can result in neuronal sensitization both in the thalamus and dorsal horns. Both pathways depend on the production of chemokines and other molecules important in the inflammatory response.
A very important molecule in the thalamus appears to be cysteine-cysteine chemokine ligand 21 (CCL21). The concentration of this chemokine is increased in the ventral posterolateral nucleus of the thalamus where secondary nociceptive neurons make connections with other neurons. The source of CCL21 is not exactly known, but two possibilities exist. First, it might be made in primary nociceptive neurons and transported up to the thalamus. Most likely, neurons intrinsic to the ventral posterolateral nucleus make at least some of it. In any case, CCL21 binds to C-C chemokine receptor type 7 and chemokine receptor CXCR3 receptors on microglia in the thalamus.[22]The physiologic response to the binding is probably the production of prostaglandin E2 (PGE2) by cyclooxygenase 2 (COX-2).[23]Activated microglia making PGE2 can then sensitize nociceptive neurons as manifested by their lowered threshold to pain.[24]
The mechanism responsible for sensitization of the central nervous system at the level of the spinal cord is different from the one in the thalamus. Tumor necrosis factor-alpha (TNF-alpha) and its receptor are the molecules that seem to be responsible for the sensitization of neurons in the dorsal horns of the spinal cord. Macrophages and lymphocytes infiltrate the spinal cord, for example, because of injury, and release TNF-alpha and other pro-inflammatory molecules.[25]TNF-alpha then binds to the TNF receptors expressed on nociceptors, activating the MAPK/NF-kappa B pathways. This leads to the production of more TNF-alpha, its release, and binding to the receptors on the cells that released it (autocrine signalling). This mechanism also explains the perpetuation of sensitization and thus allodynia. TNF-alpha might also increase the number of AMPA receptors, and decrease the numbers of GABA receptors on the membrane of nociceptors, both of which could change the nociceptors in a way that allows for their easier activation.[26] Another outcome of the increased TNF-alpha is the release of PGE2, with a mechanism and effect similar to the ones in the thalamus.[27].
Inflammatory Pain[edit | edit source]
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ 1.0 1.1 Arendt-Nielsen L, Svensson P (2001). "Referred muscle pain: basic and clinical findings". Clin J Pain 17 (1): 11–9.
- ↑ 2.0 2.1 Simons, D.G.; Travell, J.G.; Simons, L.S. (1999). Travell &amp;amp;amp;amp;amp;amp;amp; Simons' Myofascial Pain and Dysfunction: Upper half of body. Williams &amp;amp;amp;amp;amp;amp;amp; Wilkins. p. 750
- ↑ "Myocardial infarction comes with referred pain or radiating pain?". January 2, 2011. Retrieved December 26, 2011
- ↑ Hart BL (1988). "Biological basis of the behavior of sick animals". Neurosci Biobehav Rev 12 (2): 123–37.
- ↑ Marchand F, Perretti M, McMahon SB (July 2005). "Role of the immune system in chronic pain". Nat. Rev. Neurosci. 6 (7): 521–32
- ↑ DuPen A, Shen D, Ersek M (September 2007). "Mechanisms of opioid-induced tolerance and hyperalgesia". Pain Manag Nurs 8 (3): 113–21
- ↑ Chu LF, Angst MS, Clark D (2008). "Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations". Clin J Pain 24 (6): 479–96.
- ↑ Okuda-Ashitaka E, Minami T, Matsumura S, et al. (February 2006). "The opioid peptide nociceptin/orphanin FQ mediates prostaglandin E2-induced allodynia, tactile pain associated with nerve injury". Eur. J. Neurosci. 23 (4): 995–1004.
- ↑ Ikeda H, Stark J, Fischer H, et al. (June 2006). "Synaptic amplifier of inflammatory pain in the spinal dorsal horn". Science 312 (5780): 1659–62
- ↑ Tamai H, Sawamura S, Takeda K, Orii R, Hanaoka K (March 2005). "Anti-allodynic and anti-hyperalgesic effects of nociceptin receptor antagonist, JTC-801, in rats after spinal nerve injury and inflammation". Eur. J. Pharmacol. 510 (3): 223–8
- ↑ Kelley KW, Bluthé RM, Dantzer R, et al. (February 2003). "Cytokine-induced sickness behavior". Brain Behav. 17 (Suppl 1): S112–8.
- ↑ Maier SF, Wiertelak EP, Martin D, Watkins LR (October 1993). "Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin". Brain Res. 623 (2): 321–4.
- ↑ Merskey &amp;amp; Bogduk (Eds.) Classification of Chronic Pain. Seattle: IASP Task Force on Taxonomy, 1994
- ↑ Attal N, Brasseur L, Chauvin M, Bouhassira D (1999). "Effects of single and repeated applications of a eutectic mixture of local anaesthetics (EMLA) cream on spontaneous and evoked pain in post-herpetic neuralgia". Pain 81 (1–2): 203–9
- ↑ LoPinto C, Young WB, Ashkenazi A (2006). "Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine". Cephalalgia 26 (7): 852–6.
- ↑ Fitzpatrick, David; Purves, Dale; Augustine, George (2004). Neuroscience. Sunderland, Mass: Sinauer. pp. 231–250.
- ↑ Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL (1998). "Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model". Pain 75 (1): 141–55
- ↑ Wasner G, Naleschinski D, Baron R (2007). "A role for peripheral afferents in the pathophysiology and treatment of at-level neuropathic pain in spinal cord injury? A case report". Pain 131 (1–2): 219–25
- ↑ Kalous A, Osborne PB, Keast JR (2007). "Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury". J. Comp. Neurol. 504 (3): 238–53
- ↑ Zhao P, Waxman SG, Hains BC (2007). "Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21". J. Neurosci. 27 (33): 8893–902.
- ↑ Wei XH, Zang Y, Wu CY, Xu JT, Xin WJ, Liu XG (2007). "Peri-sciatic administration of recombinant rat TNF-alpha induces mechanical allodynia via upregulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: the role of NF-kappa B pathway". Exp. Neurol. 205 (2): 471–84.
- ↑ Dijkstra IM, de Haas AH, Brouwer N, Boddeke HW, Biber K (2006). "Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo". Glia 54 (8): 861–72
- ↑ Alique M, Herrero JF, Lucio-Cazana FJ (2007). "All-trans retinoic acid induces COX-2 and prostaglandin E2 synthesis in SH-SY5Y human neuroblastoma cells: involvement of retinoic acid receptors and extracellular-regulated kinase 1/2". J Neuroinflammation 4: 1
- ↑ Rukwied R, Chizh BA, Lorenz U (2007). "Potentiation of nociceptive responses to low pH injections in humans by prostaglandin E2". J Pain 8 (5): 443–51.
- ↑ Haskó G, Pacher P, Deitch EA, Vizi ES (2007). "Shaping of monocyte and macrophage function by adenosine receptors". Pharmacol. Ther. 113 (2): 264–75.
- ↑ Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005). "Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha". J. Neurosci. 25 (12): 3219–28.
- ↑ Coutaux A, Adam F, Willer JC, Le Bars D (2005). "Hyperalgesia and allodynia: peripheral mechanisms". Joint Bone Spine 72 (5): 359–71.