Electrical Stimulation - Its role in upper limb recovery post-stroke
Original Editor - Your name will be added here if you created the original content for this page.
Top Contributors - Rebecca Graham, Grant Burns, Craig Philip, Joshua Tan, Hannah Little, Rucha Gadgil, Kim Jackson, 127.0.0.1, Rachael Lowe, Admin, Venugopal Pawar, Evan Thomas, Jane Hislop, Cindy John-Chu, Carina Therese Magtibay and Dinu Dixon
Introduction
[edit | edit source]
Welcome to this online learning resource on the use of electrical stimulation (ES) to support recovery of upper limb following a stroke. This page has been created by a small group of final year physiotherapy students from Queen Margaret University as part of the Contemporary and Emerging Issues in Physiotherapy module.
Aims and Learning Outcomes
This resource aims to provide an interactive learning package for final year students and newly qualified physiotherapists to develop their knowledge and understanding of ES application for upper limb recovery following a stroke. A balance of theory, policy and evidence-base, as well as practical aspects for application of ES has been incorporated.
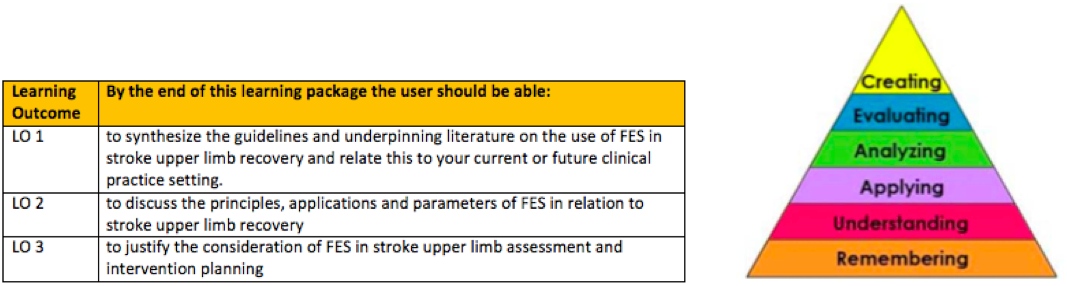
Table 1. below describes the intended learning outcomes. They have been constructed using blooms taxonomy (Figure 1.) (Anderson and Krathwohl 2000) to guide the expected level of competency, and is aligned with those expected of a newly qualified physiotherapist, described in the knowledge and skills framework KSF(DH 2004). Link to the Physiotherapy band 5 role profile can be found in the resources section at the bottom of this page.
Table 1. Learning outcomes
Layout and Approach
This package should take approximately ten hours to work through, however, this should be viewed as a guide only as people have varying learning styles and preferences. To support this the sections have been designed in a way which enables users to dip in and out to suit their needs. Users can choose to work on their own, or benefit from discussing and reflecting on the activities with others, such as peers, colleagues and managers. This choice is left to the user to match with their own preference and time-management. At the start of each section, a brief description of what will be covered is outlined and linked to the above learning outcomes.
This learning resource aims to be engaging and interactive. While synthesis and summary of the key information has been provided, the user will gain greater benefit by engaging with the directed reading, videos, activities, short quizzes and case studies that have been developed to support a deeper learning experience in line with adult learning theory (Biggs and Tang 2011). A range of material has been incorporated in the design of this package to try and suit different learning styles. VARK Learn Ltd (http://vark-learn.com) offer a tool that helps people identify their own learning preferences and maybe a helpful activity prior to commencing this resource.
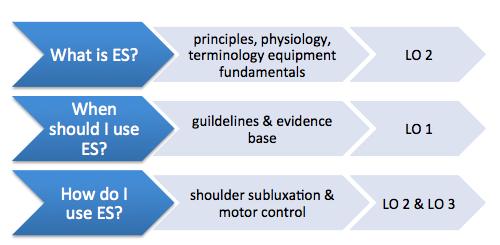
Figure 2. below outlines the content covered in the main sections of this resource
Figure 2. Outline of main sections in with mapped learning outcomes
Why this Topic?
Stroke has a large impact and burden on society (Stroke Association 2015). It is currently the 4th largest cause of mortality in the UK (Stroke Association 2015). Although trends show decreased mortality rates over the last 20 years, it is still the leading cause of complex adult disability (Stroke Association 2015). The UK has approximately 1.2 million stroke survivors, with half experiencing disability and 77% with upper limb difficulties (Stroke Association 2015). In the UK the over 65’s population is estimated to rise by over 40% in the next 17 years (AgeUk 2015), and stroke incidence is higher in this demographic. (Stroke Association 2015). This could potentially lead to even greater numbers of stroke survivors requiring support and rehabilitation from healthcare professions such as physiotherapy. Additionally, 36% of 65+ live alone (AgeUK 2015) underpinning the importance of successful rehabilitation of upper limb function if people are to maintain independence, due to its impact on performance of activities of daily living (Pollock et al. 2014).
Physiotherapists are a core member of the multidisciplinary team required to rehabilitate stroke survivors (SIGN 2010). Their main goals are to enhance people’s functional abilities, helping them improve or maintain their mobility and independence. This aligns with Scottish policy of supporting people to live independently in their homes and communities longer (The Scottish Government 2010). Physiotherapy interventions for upper limb recovery after stroke have moderate or low quality evidence, and there is insufficient data to draw comparisons as to which is most effective (Pollock et al. 2014). Therefore guidelines recommend that practice should not be limited to one approach but should be based on the need and preferences of the patient (SIGN 2010).
ES has a developing evidence base that supports its use for upper limb recovery after stroke (SSAF 2014) and the number of trials has quadrupled over the last decade (Quandt and Hummel 2014). However, current practice is varied and research shows that a lack of knowledge and skills are a key barrier to its use (SSAF 2014). This learning package aims to addresses this contemporary issue by introducing and synthesizing key literature and translating this into practical recommendations that can support clinical practice.
What is Electrical Stimulation?[edit | edit source]
Overview
ES is an assistive technology that can be used to aid the recovery of upper limb after stroke. It uses electrical current to stimulate muscle contraction via electrodes, facilitating movement of a weakened or paralyzed limb. It has been used since the mid 1960’s, traditionally to aid mobility through addressing dropped-foot, however, more recently it has been considered as a promising treatment modality for upper-limb recovery (SSAF 2014). ES has also been used in the treatment of other upper motor neuron impairments including people with Cerebral Palsy, Parkinson’s Disease, Multiple Sclerosis and spinal cord injury (Odstock medical 2006).
FES Uses
Several uses and benefits have been investigated regarding ES use in stroke upper limb recovery. These include strengthening weak muscles, increasing range of motion, reducing spasticity, improving motor control, reducing shoulder subluxation, reducing pain associated with shoulder subluxation and spasticity, improving sensory and proprioceptive awareness, and improving effects of botulinum toxin for management of spasticity (Odstock medical 2002; SIGN 2010, Foley et al. 2013). Neuroplasticity is a key concept underpinning stroke recovery and it is the ability of the brain to adapt and form new neuroconnections (Van Wijck and McBean 2013). By forming these new synapses, motor-skills can be relearned and concepts of sensory-motor learning are founded on this premise (Schmidt xxxx). Following stroke there is evidence that the brain has a period of hyper-excitability within the first weeks after stroke (Butefische xxxx) and it is hypothesised that by affert stimulation central reorganisation can be enhanced by stimulation through movement which ES may be able to facilitate (Meilink 2007). Additionally there is large predictive probability (90%) of return of upper limb function decided within the first 5 weeks, indicating a critical window for influencing recovery. As will be reviewed in the following section (When should I use ES?), evidence supporting use of ES is not conclusive (SSAF 2014, Pollock et al. 2014, Howlett et al. 2015). This resource focuses on two key areas; shoulder subluxation and motor control as these are the only two supported by UK guidelines (SSAF 2014; NICE 2013; SIGN 2010; RCP 2012).
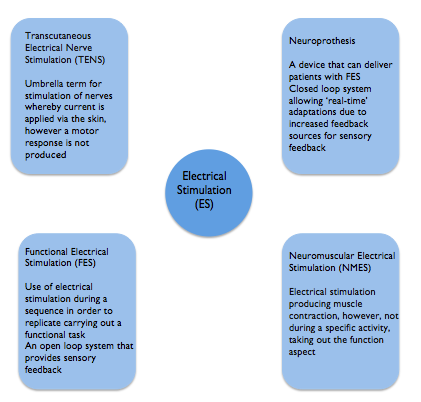
Terminology
There are various terms used within the literature for ES. Although each has a different meaning they are frequently used synonymously which can make understanding and comparing different studies challenging (SIGN 2010). Highlighted below are the most common terms encountered in the literature. This learning resource has focused on ES used to support upper limb recovery which is delivered predominantly by Neuromuscular Electrical Stimulation (NMES). Functional electrical stimulation (FES) and neuorprothesis are forms of NMES. Transcutaneous electrical nerve stimulation (TENS) has traditionally been used for analgesic purposes and does not elicit a motor response (Robertson et al. 2006; SSAHPF 2014) although it has also been used in studies to promote sensory feedback (SSAHPF).
One distinction made is the use of ES for therapeutic purposes such as to aid motor learning with the intention of a carryover effect beyond treatment. Functional electrical stimulation on the other hand is aimed at providing direct benefit to aid a task at the time of wearing and is used in an orthotic manner (Quandt and Hummel 2014).
Physiology
ES uses electrodes to activate contraction and relaxation of muscles that have been affected by an upper motor neuron lesion. Motor-units are electrically stimulated by depolarization of motor axons, or terminal motor nerve branches. When depolarization reaches threshold, an action potential occurs due to sodium flowing from the extracellular to intracellular space. This action potential propagates along the nerve fibre axon to the muscle via the neuromuscular junction resulting in muscle contraction (Neo stroke network 2015). As an intact connection between the ventral horn and muscle is required for successful action potential propagation to reach the muscle, ES is not suitable for lower motor neuron lesions (Odstock 2015). In ES it is the nerves that are stimulated rather than muscle, as they require a lesser current of that needed to trigger muscles directly. Influencing factors include distance from electrode to nerve fibre, size of motor unit, and surrounding tissue will all impact the number and type of motor units activated (Gorman and Peckham 2014).
ES engages the patient and delivers feedback of a sensory and visual nature, beneficial for stroke patients during their recovery and promotes motor re-learning (Dobkin and Dorsch 2013; Kawashima et al. 2013; Howlett et al. 2015; Odstock medical 2015). Disuse atrophy is a common secondary complication of stroke and can result in muscle fibre changes (Pollack et al 2014). ES may also be able to reverse this by bringing about muscle fibre changes over the course of treatment, with type II glycolytic fibres reverting to type I oxidative skeletal muscle fibres (Gorman and Peckham 2014). Type II fibres generate greater forces but fatigue more quickly, whereas type I fibres produce lesser force but are more fatigue-resistant (Sheffler and Chae 2007).
Motor unit recruitment and Fatigue
In normal physiology, nerve fibre recruitment occurs as described by the Henneman size principle of voluntary motor unit recruitment, whereby nerves with the smallest diameter will be recruited first. When using ES, the reverse of this happens, with the largest diameter neurons being recruited first due to a lower nerve stimulus threshold (Sheffler and Chae 2007) which can lead to fatigue. However, it has recently been established that it is in fact an uncoordinated method of recruitment, with no order, that is evident in ES (Quandt and Hummel 2014).
One limitation of ES is that muscles can fatigue (Thrasher et al. 2005) and that the higher frequency selected, the quicker muscle fatigue will set in. To address this, the lowest frequency possible to achieved the desired outcome (tonic muscle contraction) should be used, and relies on the clinical expertise to achieve this (SSAF 2014). Once fatigue has set in, there is argument for and against whether muscle strengthening can occur. It has been suggested that when fatigued, that no advantages can be gained from additional stimulation and therefore fatigue should be prevented if possible. Opposing arguments suggest that strengthening can only be achieved if the muscle fibre is worked to its maximum. Clarifying the cause of fatigue is important. Strengthening can occur if fatigue is within the muscle fibres due to cellular processes being activated, however if fatigue has resulted from neurotransmitter depletion or propagation failure, then the muscle will not be strengthened as the fibre is not being stimulated sufficiently (Robertson et al. 2006).
ES Devices and Parameters
ES systems include three components: the control, an electrical stimulator and electrodes which connects the ES with the nervous system (Gorman and Peckham 2014).
Electrodes
Application of ES is done via electrodes, which generate the electrical field through either surface or percutaneous electrodes (Ewins and Durham 2008).
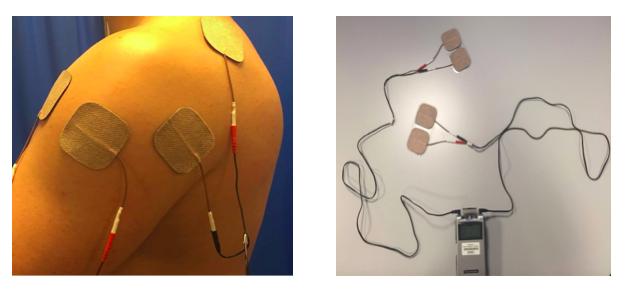
Surface electrodes are placed directly onto the skin over the nerves and are the most common Sheffler and Chae 2007). As well as stimulating muscle, surface electrodes may also be used to achieve a reflex action (Ewins and Durham 2008). Parameters of ES needed when using surface electrodes can differ depending on factors such as material of the electrodes, placement and surface area (Sheffler and Chae 2007). They are non-invasive and relatively inexpensive and are suitable for use across a wide variety of settings and by therapists and patients, promoting independence and self-management (SSAF 2014). However, targeting contraction of small individual muscles can be difficult, and often activation of deeper muscles requires superficial muscles to be activated first. Surface electrodes have also been reported to cause pain for some patients when compared with percutaneous electrodes (Popaviz 2003). Percutaneous electrodes penetrate through the skin into the muscle via hypodermic needles or can be completely implanted whereby stimulation is received from an external unit (Sheffler and Chae 2007). They can address the difficulties faced with surface electrodes by being able to target deep muscles and as they use lower currents, are reported to be less painful (Sheffler and Chae 2007). However, factors of cost and practicality need to be taken into consideration (Ewins and Durham 2008). In line with the SSAF (2014) recommendations, this resource focuses on the surface mounted electrodes as they are inexpensive and most commonly used, therefore best suited to this learning resources target audience as it covers the equipment they are most likely to encounter in practice.
UniPolar vs Bipolar
Two electrodes are required (active and indifferent) to generate a flow of current, however these can be unipolar or bipolar in configuration. Unipolar is when one electrode is more active than another due to their sizes. The active electrode is typically smaller and placed near the nerve to be stimulated with the indifferent electrode placed over less excitable tissue such as fascia. In multi-channel configurations there are several active electrodes but only one indifferent electrode is required (Robertson 2006). Biploar electrodes are both the same size meaning the current at each site will be equal. Both active and indifferent electrodes are placed close to the stimulated nerve and in multi-channel configurations there is an indifferent electrode for each active. Bipolar systems enable greater targeting of muscles (Robertson 2006).
Cyclical, button & EMG-triggered
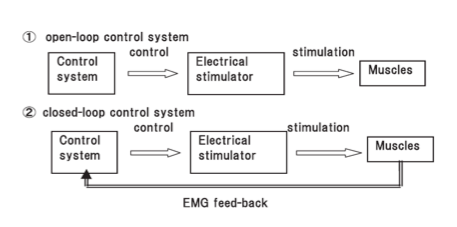
One other variable is whether the timing of when electrical stimulation is active is via a button, cyclical or EMG-triggered system. The button method is a manual form of ES and requires the user to actively press a switch to activate the stimulation. Cyclical means that the timing and sequence is predetermined by selection of an appropriate programme on the device and this is sometimes just referred to at NMES (SSAF 2014). Alternatively, the EMG triggered system uses sensors built in to the active electrode to determine when voluntary muscle contraction is above a preset-threshold which then triggers the ES (SSAF 2014). It has been suggested that this method promotes greater user involvement promoting neuroplastic changes (Quandt and Hummel 2014) and leads to better outcomes (deKroon 2005; Hara et al. 2008). Typically this is called EMG-triggered ES to differentiate from cyclical (SSAF 2014). Cyclical or predetermined programs are considered open loop systems that rely on the user to turn-on/off whereas EMG-triggered are classified as closed-loop systems as the EMG trigger determines on/off timing.
Open and closed loop ES systems (Hara et al. 2008)
Parameters
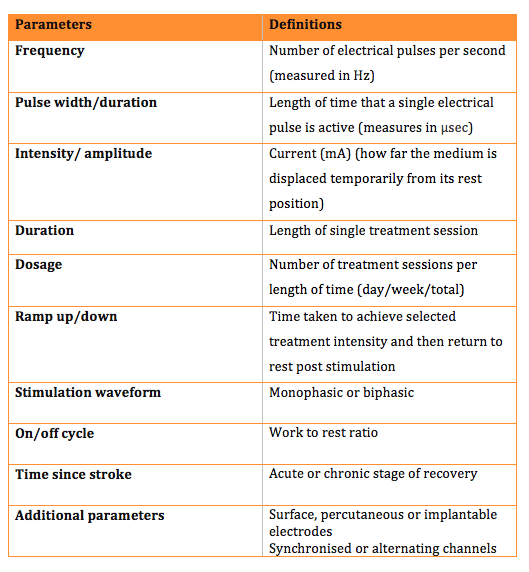
There are various parameters that can be adjusted on ES devices to help tailor the electrical field effects to the patient and its intended application. The table below outlines each parameter and its definition. It is worth noting that not all ES devices allow individual control of all parameters and many offer a choice of pre-determined programmes.
What's Available?
Odstock medical is one of the main suppliers of ES devices, which vary in design and parameters available. The odstock 4 channel stimulator kit and microstim 2V2 kit are both simple to use in order to allow regular activity in the home environment. They have this in common however differ in output as the 4 channel stimulator can provide alternating or continuous output, unlike the microstim. The odstock website has further images and descriptions of the ES kits available. Please visit the website www.odstockmedical.com to familiarise with the options available.
When should I use Electrical Stimulation[edit | edit source]
Guidelines[edit | edit source]
The following section provides an overview of UK guideline recommendations of electrical stimulation for upper limb rehabilitation. It primarily address learning outcome 1.
The information and activities in this section will draw from the following guidelines:
- SIGN Guideline 118 - Management of patients with stroke (SIGN 2010)
- NICE Clinical Guideline 162 – Stroke Rehabilitation (NICE 2013)
- Royal College of Physicians – National clinical guideline for stroke (RCP 2012)
Overall there is general consensus and robust evidence to support the use of ES in the management of shoulder subluxation and this features in all listed guidelines.
However the findings for ES are not ubiquitous for other uses (motor control, spasticity, shoulder pain), although motor control and learning does have a developing evidence base and this is reflected in the 'How do I use ES' section where guidance is provided for both shoulder subluxation and motor control as this reflects the main areas of practice currently within the NHS (SSAF 2014).
SIGN Guideline 118 - Management of patients with stroke (SIGN 2010)
Activity: Read SIGN 118: http://www.sign.ac.uk/pdf/sign118.pdf
- p. 19, section 4.3.1 & 4.3.2. – Upper limb function
- p. 31 & 32, section 4.9.1 & section 4.9.3 – Post-stroke spasticity
- p. 34 & 35, section 4.10.1 & 4.10.3 – Prevention and treatment of shoulder subluxation
- p. 36 & 38, section 4.12.1 & 4.12.7 – prevention of post-stroke shoulder pain
- p. 39 & 40, section 4.13.1 & 4.13.6 and 4.13.7 – treatment of post-stroke shoulder pain
Summary Recommendations
The following recommendation is relevant to the use of ES in the rehabilitation of upper limb.
Section 2.2: Key intervention and treatment recommendations:
“Electrical stimulation to the supraspinatus and deltoid muscles should be considered as soon as possible after stroke in patients at risk of developing shoulder subluxation. (SIGN 2010, p. 6)”. Grade A
Section 4.3 Electro-stimulation for Upper Limb
ES currently falls into the ‘insufficient evidence’ category for upper limb function.
Five systematic reviews (SR) were evaluated along with 4 additional RCTs. The findings were inconsistent and there is insufficient high quality evidence to make recommendation for/against its use. It was noted that there was limited evidence to show there may be some beneficial outcomes in upper limb however it was insufficient to make a recommendation. The most recent paper included in this review is from 2008, however the majority of the papers, including the five SR’s, included data that was 10 years old or greater, and this needs to be considered when reading this guideline. A SR published this year (Howlett et al. 2015) has shown positive effects of FES on upper limb function after stroke and recommends the consideration of FES in upper limb rehabilitation, however it identifies the timing as a critical factor. Additionally due to the quality and variation of the studies included, it was unable to determine the clinical significance of the finding and ultimately recommended further research was required.
Section 4.9 Post-Stroke Spasticity
Routine FES falls into the insufficient evidence category for treating spasticity, defined as “intermittent or sustained involuntary hyperactivity of the skeletal muscles associated with an upper motor lesion (SIGN 2010, p. 31).”
Only One SR from 1996 was found. It included 4 RCT’s , all poor quality and only one was upper limb (wrist). Although strength gains were reported there was no functional improvement or measured reduction in tone.
A more recent RCT (Hara et al. 2006) investigated the effects of FES combined with phenoyl injections and benefits were seen to spasticity in finger flexor muscles, however because of the hybrid design there was no way to isolate the FES benefits from this study.
Section 4.10 Shoulder Subluxation
ES is the only recommended intervention for early treatment of shoulder (or at risk of) subluxation. 3 SR’s support the recommendation. One limitation is that the majority of literature is prior to 1999 with the most recent SR published in 2003 therefore this information could be dated although the recommendation is still supported by more recent literature Guidelines (SSAF 2014).
The guideline recommends targeting both the supraspinatus and deltoid muscles as soon as possible. (Grade A)
Section 4.12 Shoulder Pain Prevention
Several SRs were included in this section. Overall the conclusion was that FES did not prevent shoulder pain in patients who demonstrated upper limb weakness, despite some papers reporting improved function, shoulder rotation and subluxation. These findings included a 2002 Cochrane review of FES & TENS, a 2001 SR of Electrical stimulation, a large RCT (n=176) of NMES in 2006 and a more recent RCT (n=23) of FES and as a result the recommendation was considered grade A. One difficulty reported was distinguishing between the definitions and overlap of FES, NMES & ES.
Section 4.13 Shoulder Pain Treatment
Although the evidence to preventing shoulder pain was conclusive, the use of FES, in treatment of shoulder pain stated there is insufficient evidence to support or refute its use. A SR in 2000 and 2002 were inconclusive although the most recent did find reduction in pain-free abduction from FES 2 months after stroke. Intramuscular ES has also been reviewed and showed benefits compared with sling but it was unclear whether the benefits were from ES or from adverse effects from the sling.
Clinical Guideline 162 NICE (2013)
Activity: Read Clinical Guidline 162: http://www.nice.org.uk/guidance/cg162/evidence/full-guideline-190076509
- p. 31, section 90. to 94 – Electrical stimulation: upper limb
- p. 437-438, 13.4.2 – Recommendations and link to evidence
NICE CG162 ES Recommendations (NICE 2013. P. 437).
- “Do not routinely offer people with stroke electrical stimulation for their hand and arm.
- Consider a trial of electrical stimulation in people who have evidence of muscle contraction after stroke but cannot move their arm against resistance.
- If a trial of treatment is considered appropriate, ensure that electrical stimulation therapy is guided by a qualified rehabilitation professional.
- The aim of electrical stimulation should be to improve strength while practicing functional tasks in the context of a comprehensive stroke rehabilitation programme.
- Continue electrical stimulation if progress towards clear functional goals has been demonstrated (for example, maintaining range of movement, or improving grasp and release).”
NICE Guidelines Summary
There were no cost effectiveness studies identified and the evidence was based on 19 RCTs. There was positive evidence for FES in many studies however confidence was generally low or very low. Low numbers of participants and high likely hood of bias limited confidence. Heterogeneity across trials was also significant. There is a recommendation to investigate the topic further and this is a consistent finding with the SIGN guidelines.
There were no significant risks in using electrical stimulation and where arm function has some muscle contraction present but not able to lift against gravity may be the best target group for results with FES. FES should be used to support muscles strengthening in the function task training and practice typically as an adjunct approach.
Royal College of Physicians – national clinical guidelines for stroke 4th edition (RCP 2012)
Activity: Read RCP national clinical guideline for stroke 4th edition : https://www.rcplondon.ac.uk/file/1299/download?token=mcyQFjEq
- p. 90, section 6.13 – Neuromuscular Electrical Stimulation
- p. 94-95, section 6.19.2 – Shoulder pain and subluxation
'Key Recommendations'
- “Therapeutic electrical stimulation for treatment of the upper and lower limbs following stroke should only be used in the context of a clinical trial” (RCP 2012, p. 157).
- “Any patient who has developed, or is developing, shoulder subluxation should be considered for functional electrical stimulation of the supraspinatus and deltoid muscles “(RCP 2012, p. 158).
How do I use Electrical Stimulation[edit | edit source]
General Considerations[edit | edit source]
Considerations
ES can be applied by qualified health professionals; including physiotherapists and occupational therapists who are competent in its use. Although this learning package gives a theoretical overview of ES we advise that practical training is performed before applying this as a treatment (Allan and Goodman 2014).
The checklist below details what training is required prior to use of ES.
ES training checklist
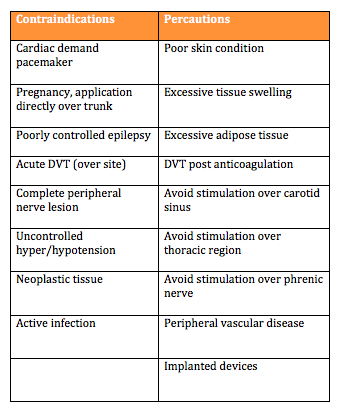
Contraindications and Precautions:
To ensure patient safety it is important to consider the contraindications and precautions before using an ES device. Understanding these will help make an informed decision regarding the use of ES as a treatment.

Page 3Using Electrical Stimulation A Guideline for Health Professionals
Page 2 Referral Criteria OML Learning Through Technology
The above documents highlight a variety of contraindications. Detailed below is a summary table of the main contraindications and precautions identified by the Scottish Stroke Allied Health Professionals Forum.
Percautions and contraindications quiz
What do Patients need to know?
Prior to commencing treatment the patient needs to give verbal consent. After they have given consent it is important to provide the patient with information regarding ES. They should be informed of the expected skin sensation and that it may uncomfortable, but they should not experience any pain. Patients should also be told what to do if they experience these sensations and how to work the ES device if a patient or carer is able to adjust it. Providing an instruction manual in lay terms may be beneficial for the patient. Contraindications and precautions should be highlighted to the patient with relevant information explained to them. After providing the patient with this information, it should be documented in the patient notes (Allan and Goodman 2014).
Outcome Measures: There are a variety of outcome measures that can be used in the monitoring of upper limb recovery and function post stroke, which could be used to track progress of patients using ES. Two of these outcome measures will be outlined below.
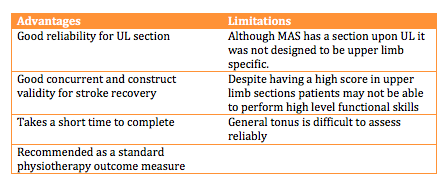
Motor Assessment Scale(MAS):
The MAS is an outcome measure which focuses upon functional motor activities. It has specific sections for Supine to side lying, Supine to sitting over side of bed, Balanced sitting, Sitting to standing, Walking, Upper-arm function, Hand movements, Advanced hand activities and general tonus. Each of these are scored from 0-6. A score of six is the optimal motor score in each area, with a score of 48 being available in total (CARR et al 1985a). View the MAS outcome measure here: File:Motor Assessment Scale.pdf
Wolf Motor Function Test:
The Wolf Motor Function Test is a timed outcome measure which aims to assess how quickly functional upper limb tasks can be performed. Patients are given 120 seconds to complete each task before the task is marked as incomplete. Each of these are scored from 0 to 5. Five is the best score achievable for each task, with 75 being the maximum score available (WOLF ET AL 2005a).
VIDEO
DOCUMENT
Shoulder Subluxation[edit | edit source]
In this section the reader will have the opportunity to develop their knowledge and understanding relating to ES and it’s uses for shoulder subluxation post stroke.
This section will cover:
• Shoulder subluxation in stroke
• How does FES aid in Shoulder Subluxation?
• Application
• Dosage and Parameters
• How would I identify patients at risk?
• Outcome measures
• Summary
• Key note
• Evidence based
• Case study
• Further Resources
Shoulder subluxation in stroke
• Shoulder subluxation is a common problem amongst patients with extreme muscle weakness and limb inactivity. This occurs mainly due to the effect of gravity, stretching inactive soft tissues (Carr and Shepard 2003).
• Weakness of the shoulder musculature can result after a stroke, often leading to subluxation due to the muscles being unable to hold the humerus within the socket of the glenohumeral joint, further assisting gravity in pulling the humerus into an abnormal position.
• A reliable measure to test for a subluxed shoulder, is by using callipers to measure the subacromial space between the acromion and humeral head (Boyd & Torrance 1992).
For further information of shoulder subluxation in stroke please read Carr and Shepard 2003 pages 195 – 197 & 204
Definition:
Shoulder subluxation is described as inferior glenohumeral joint displacement and is a very common secondary musculoskeletal impairment in the upper limb post stroke (Ada and Foongchomcheay 2002).
Incidence of shoulder subluxation:
Shoulder subluxation occurs in 17 – 81% of patients following a stroke and has been reported as the main cause of shoulder complications (Manigandan et al. 2014). Shoulder subluxation has a high occurance rate in hemiplegic patients.
Take 10 mins to read the pathophysiology of Shoulder Subluxation in Stroke:
➢ Section 11.3
➢ Pages: 6 – 11
➢ http://www.ebrsr.com/sites/default/files/Chapter11_HemiplegicShoulder_FINAL__16ed.pdf
How does FES aid in Shoulder Subluxation?
• There is strong evidence supporting the use of FES in clinical practice in order to treat shoulder subluxation. whereby it directly stimulates the nerves and not the muscle fibres.
• Electrical stimulation should be started as early as possible i.e. initiated acutely post stroke as part of best practice for those patients who are at risk of developing subluxation due to paralysis of shoulder muscles after stroke (Ada and Foongchomcheay 2002). It also has benefits for patients in the chronic stages of stroke such as reducing the distance between the acromion and the humeral head (EBRSR 2013).
• FES also seems to helps improve function, muscle tone, joint alignment and sensory deficits (Price & Pandyan 2001).
• Functional electrical stimulation is applied to structures that aid in maintaining the position of the head of humerus in the glenoid fossa such as the supraspinatus and deltoid muscles (Vafadar et al. 2014). The long head of biceps should also be taken into consideration for patients post stroke to help minimise the risk of shoulder subluxation (Manigandan et al. 2014).
• Stimulation of the supraspinatus alone would be inadequate to maintain the humerus position in the shoulder (Kobayashi et. al. 1999)
• FES may aid in minimizing shoulder subluxation and could further be used as a preventive measure, however it does not seem to reduce pain (EBRSR 2013).
How do I identify a patient at risk of a shoulder subluxation?
An audit by (Macdonald 2013) reported:
• That patient with a subluxation was precisely identified as at risk by using the predetermined criteria (Appendix B2).
• Great majority of patients presented with a subluxation within the first week of admission.
• Patients who did not develop subluxation exhibited an increase of decreased sensation and proprioception compared to those with shoulder subluxation.
• 100% of patients who developed subluxation had low tone. Further stating that patients with either flaccidity or low tone around the shoulder with reduced active ranges of motion could be considered a risk.
• However she mentioned that only 50% of patients with the risk of shoulder subluxation would be suitable electrical stimulation and therapists should use their own clinical reasoning and judgment to select such eligible patients.
Application:
Before applying FES to the upper limb to assist reduction of shoulder subluxation, you should consider the movement you wish to illicit and the structures involved in this movement.
Below are some illustrations of FES being used with slight variations.
The settings below are by Odstock Medical Limited who are one of the main distributors of FES in the UK.
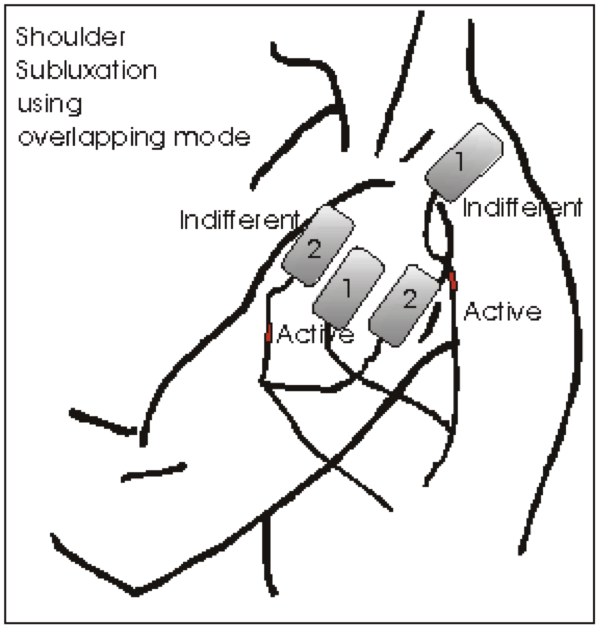
Reducing Shoulder Subluxation
2 pairs of electrodes required
Placement of pair 1: Supraspinatus & Middle of Deltoids
Placement of pair 2: Anterior & Posterior Deltoids
All 4 electrodes should fit under the hand of the clinician over the patient’s shoulder. When adjusting the current to relocate the position of the humerus, extensive shoulder abduction should be avoided.
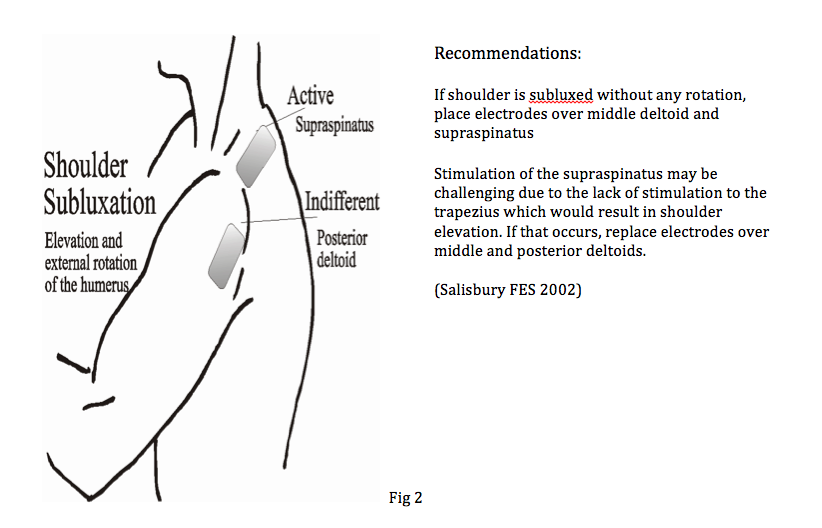
Reduction of shoulder subluxation with external rotation
This is suitable for patients with significant anterior subluxation/ internal rotation of humerus.
2 pairs of electrodes required
Placement of pair 1: Supraspinatus & Middle of Deltoids
Placement of pair 2: Teres Minor & Posterior Deltoids
You can adjust which electrodes is active or indifferent depending on your patient’s needs. This set up should relocate the humerus more posteriorly.
Current levels maybe too high when shoulder is brought into elevation and you should adjust the current where appropriate to achieve humeral relocation.
Take 5 minutes to watch the tutorial on shoulder subluxation and skip video to 1.45 to commence shoulder subluxation video.
https://www.youtube.com/watch?v=yWMzzY_Zrv0#t=436
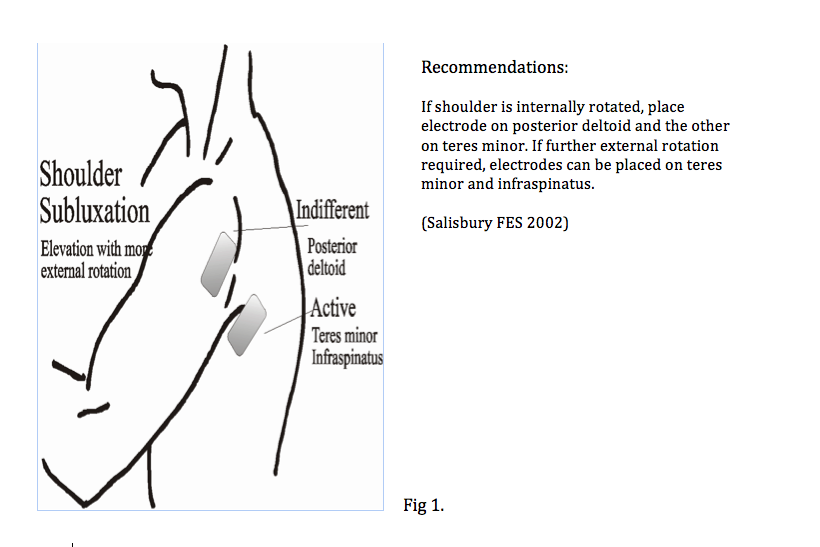
• For Figures 1 & 2 choose which electrode to make active (Strongest effect) i.e. if active electrode over teres minor causing extensive external rotation, reverse the polarity.
• Dual channels of stimulation can be applied or alternation of electrode positions.
Take note:
1. Different electrode sizes available, use whereby appropriate.
2. Electrodes should strictly be a one patient use.
Dosage and Parameters:
• Scottish Stroke AHP forum has reported that subluxation appears to arise during the flaccid period, which is the first 3 weeks post stroke. Shoulder subluxation is less likely to occur if the supraspinatus has developed some movement.
• Further evidence has also shown that early application of FES preferably within the first 48 hours post stroke is vital in preventing shoulder subluxation (Linn et. al. 1999, Fil et. al. 2011).
• FES treatments also show no further improvements after 12 months and the results remained the same 12 months later (Chantraine et. al. (1999)).
• Before commencing an FES treatment on a patient, it is important to consider the types of parameter settings available and decide upon the most appropriate setting for your patient. The different types of settings used can provoke various responses from patients.
Types of parameters to consider:
1. Frequency
2. Pulse width
3. Amplitude (intensity)
4. Duration of treatment
5. Dosage i.e. number of treatments a week
6. Ramp/ Ramp down
7. Type of wave form
8. On/off cycle
9. Structures stimulated
10. Time post stroke
This was taken from the Scottish Stroke AHP forum.
For more in depth information on the different types of settings, please read pages 75 – 77 from the Scottish Stroke AHP forum
The link to this document can be found below:
http://www.chss.org.uk/documents/2014/10/electrical-stimulation-consensus-statement-ssahpf-pdf.pdf
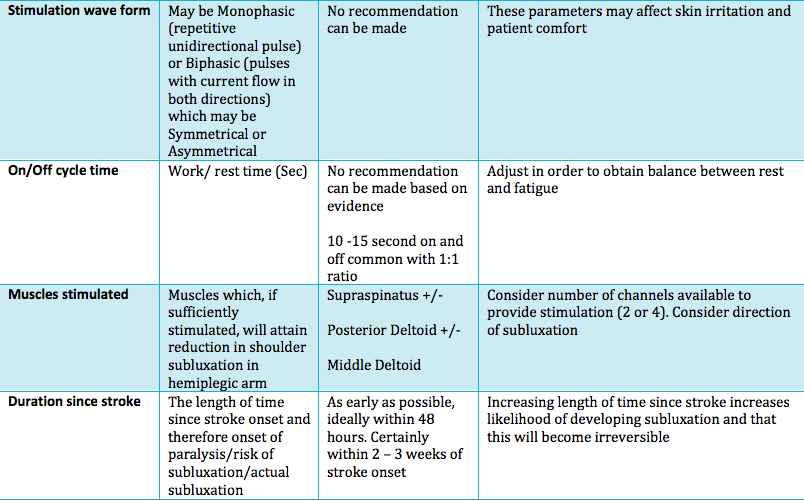
Summary of main parameters outlined below:
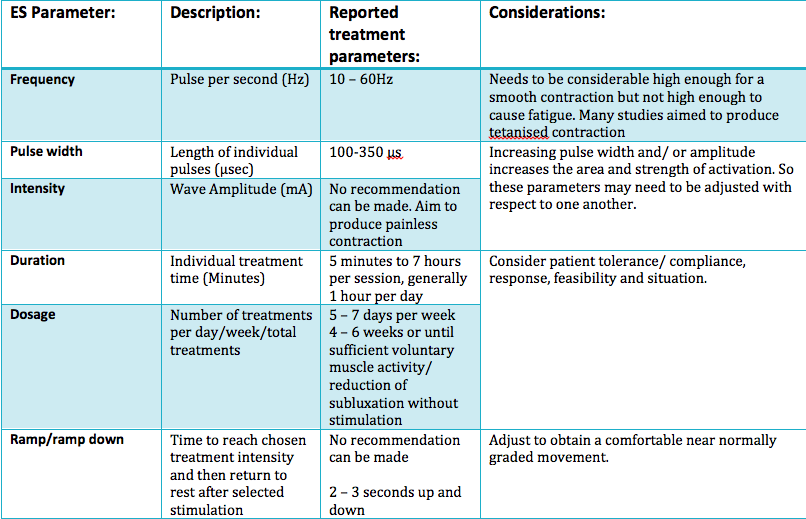
Frequency:
In order to determine the amount of muscle activity generated, the frequency choice of most authors were between 10 and 60 Hz. Whereas some used a range of frequencies to produce a tetanic contraction catered to the individual (Baker, Parker 1986). However it was reported that only frequencies above 30Hz were sufficient to eilicit muscle activity due to the need to generate enough force to counteract the inferior subluxation (Ada and Foongchomcheay 2002).
Pulse amplitude and pulse width:
Many authors did not state the pulse width or justify the choice, however, the general consciences reports of values varying from 100µs and 350µs. Factors such as muscle fatigue and patient comfort should be taken into account Kroon et. al. (2005). Though it may be the tweaking of the 3 variables that generates the most important factor, which is a visible muscle contraction. However, further investigation is required to uncover the full effects of the parameter settings of electrical stimulation on shoulder subluxation.
Length of treatment:
The evidence for length of treatment showed substantial inconsistency towards the overall. The evidence was synthesized Ada and Foongchomcheay (2002) and recommended that electrical stimulation be initially applied 1 hour per day initially and gradually increased to 6 hours per day. The evidence is unclear in the case whereby subluxation has already transpired.
Patients should continue with the treatment until they have a score of more than four on the motor assessment scale (MAS) Ada and Foongchomcheay (2002). However this was in adjust with improved levels of motor control. Correspondingly, patients who scored two or more on the motor assessment scale (MAS) did not develop shoulder subluxation Linn et. al. (1999). Therefore this could be used as an outcome measure when treating patients. Furthermore, the evidence suggested that improvements of subluxation did not change from 12 – 24 months but improved within the first 12 months of treatment.
Waveform, ramp times and on/off cycle time:
The evidence showed inconclusive waveform, ramp times and on/off cycle time and that there was no definitive guidelines on the specific type of parameters towards treating shoulder subluxation. However, the general conscencious is that electrical stimulation should be considered during the acute stages, ideally the first few days post stroke in the flaccid period where there is a high risk of subluxation due to significant muscle weakness on top of conventional therapy.
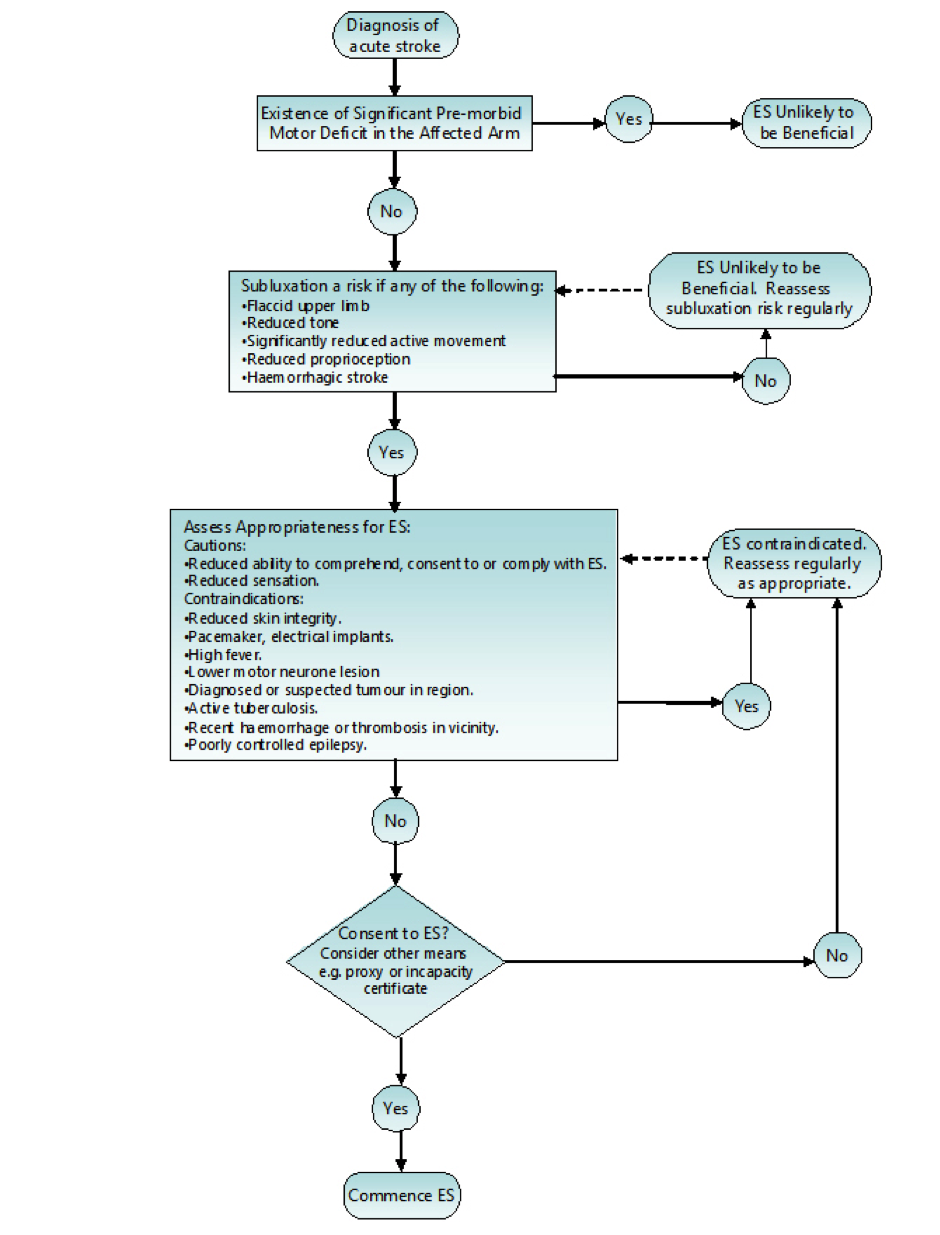
Below is a FES pathway in the usage FES to prevent shoulder subluxation post acute stroke
(Scottish stroke AHP forum 2014)
Outcome Measures:
• Motor Assessment Scale (MAS) is one of the outcome measures used to gauge a patient’s functional ability and thus feed backing to clinicians if FES is necessary. However, there is limited evidence to suggest that it is the outcome measure to be used.
To familiar yourself with the motor assessment scale, click here.
http://www.rehabmeasures.org/Lists/Admin%20fields/Attachments/924/Motor_Assessment_Scale.pdf
In Summary:
The use of FES as a treatment for preventing shoulder subluxation and those having shoulder subluxation post stroke is advocated (Intercollegiate Stroke Working Party 2012, Scottish Intercollegiate Guidelines Network (SIGN) June 2010). Furthermore, early application of FES in adjunct of traditional therapeutic treatments have proven to be more superior to conventional therapy alone. Subluxation tends to occur within the flaccid period in the first 3 weeks post stroke.
The main muscles targeted for stimulation are supraspinatus and deltoids however the evidence varies with regards to which deltoid fibers prove most beneficial. It may depend on the type of subluxation your patient may have i.e. anterior or inferior subluxation. FES should be commenced for one hour per day initially and subsequently increased to 6 hours per day (Ada and Foongchomcheay (2002).
The recommended dosage of FES is that patients should continue with the treatment until they have a score of more than four on the motor assessment scale (MAS) (Ada and Foongchomcheay (2002). However this was in adjust with improved levels of motor control. Correspondingly, patients who scored two or more on the motor assessment scale (MAS) did not develop shoulder subluxation Linn et. al. (1999). Therefore this could be used as an outcome measure when treating patients.
Key Note:
• Clinical application and parameters for shoulder subluxation and motor control vary and are different.
• Early application of FES may result in a substantial decrease in subluxation as compared to late/chronic stages of subluxation.
Evidence based/Conclusion:
There is increasing more evidence supporting the use of FES in shoulder subluxation post stroke. Currently, there is good quality evidence to show that FES should be considered during the acute stages of stroke being it either used for the prevention or treatment of subluxation. However, further research is required towards the feasibilities of the device’s parameters due to the variations of study designs and treatment constraints used.
Case Study:
To review your learning, we have developed a case study of Mr Moses to help synthesis the information previously mentioned.
Mr Moses is a 70 year old retired male living with his wife. He suffered a left sided stroke three weeks ago and has been diagnosed with right sided hemiplegia. It was noted through an initial physiotherapy assessment that due to the hemiplegia there was a significant reduction of active shoulder movement and upon further examination of the shoulder the patient was showing signs of subluxation, however a definite diagnosis of the shoulder being subluxed was never completed.
In addition, due to the stroke, Mr Mose’s cognition has been affected and therefore his wife now has power of attorney. Medical staff also noted that the patient’s overall skin condition is relatively poor in adjunct with oedema of his right hand. A colleague has approached you to help with the diagnosis and for some advice due to the lack of improvements from conventional physiotherapy treatments.
You are the clinical specialist in treating patients with FES. Would FES be beneficial towards this patient?
Activity:
Looking back at the case study above;
• Can you name a reliable measure to aid in measuring a subluxed shoulder?
Answer: Callipers by measuring the subacromial space between the acromion and humeral head.
• Identify some contraindications or cautions if any?
Answer: Poor skin condition, Oedema of right hand.
• Could you recommend a potential treatment parameter for Mr Moses?
Answer: See recommended parameters of FES pages 17 – 18 from the Scottish Stroke AHP forum.
• Which muscles would you place the electrodes on and why?
Answer: Supraspinatus, Middle or Posterior deltoid due to these muscles being the main structures in maintaining humeral position in the glenoid fossa.
8 months on, Mr Moses has made a significant recovery and is being reviewed for discharge. However, his wife is concerned about him returning home, as she is keen for him to continue progress with the use of FES as she has seen the benefits this has. She has approached you to enquire about purchasing an FES device independently to continue in Mr Mose’s rehabilitation and self management.
• Now have a think about some of the requirements you would expect from an FES device, which would enable a patient to use at home.
Answer: Easy to use, Inexpensive, Easy to charge, Suitable for unsupervised use, Light weight and compact, Easily cleaned, Not for single person use.
• Having read about the possible interventions and evidence, reflect upon a situation whereby, a patient has informed you that he would like to purchase an FES device for home use even though he has been using FES for more than a year. What advice would you give this patient and why?
Answer: I would advice this patient to reconsider buying an FES device as the evidence states that there is minimal to no change in improvements past the 12 month period. Therefore there would not be much benefit in buying the device due to the limited recovery of shoulder subluxation post stroke.
Motor Control and Recovery[edit | edit source]
Introduction:
It is often common for individuals to be left with a motor control deficit on their hemi-paretic side following a stroke. Many sufferers are left with controlled flexor synergy. This can cause difficulties when performing activities of daily living.
Electrical stimulation (ES) in patients with motor function impairment of the upper extremity has been employed as a rehabilitation modality for many years. In order for ES to beneficial as a treatment for motor control, it is reported that patients should have some degree of movement.
NICE (2015), suggest that patients should be able to hold a contraction but may not be able to move their arm against resistance. The proposed mechanism for upper limb motor control ES is to strengthen the elbow, wrist and finger extensor muscles, reduce the spasticity of the antagonist muscles and help to promote nueroplastic changes (Sailsbury 2002, Module 10). However, there is a limited evidence indicating that repeated muscle activation using ES may lead to improvement in voluntary motor control and providing a carry over effect (Quandt and Hummel 2014).
How does ES aid Motor control?
It is understood that when a muscle contraction is produced by electric stimulation, a whole range of sensory inputs are produced. This includes the direct sensation from the stimulation and proprioceptive feedback from joints, tendons, muscles and mechanoreceptors.
This causes a significant increase in the activity along the intact pathways to the cortex, stimulating the production of new synpatic connections (Taylor et al. 2002). The increased level of motor neuron excitation will also make it easier for weak descending inputs to activate the motor neuron and therefore help to produce a voluntary contraction (Quandt and Hummel 2014). When using ES to improve motor function it is often useful to combine muscles to produce a larger pattern of movement, similar to the combination of movements in ADL’s.
Appplication
Typically there are three main focuses of ES for upper limb:
• elbow extension
• wrist, finger and thumb extension
• reaching actions.
Overall the placement of the electrodes is key to achieving a comfortable, effective movement for the patient. It is important to ask the patient to assist with the movement, however this voluntary effort must not be so great that it causes a rise in spasticity and inhibits the desired movement.
The images below indicate the electrode placement and the functional movement they help to produce.
Elbow Extension:
• The triceps can be activated placing an active electrode over its motor point and the indifferent over the tendon at the elbow.
• Due to the size of the muscle it is useful to use larger electrodes, which may help produce a more effective movement.
• Practicing ‘table polishing’ by sliding the hand over a table using a cloth to reduce friction can be useful.
PICTURE
Wrist, finger and thumb extension:
• This is best achieved by stimulation of the radial nerve, which produces an extensor pattern.
• It is often a problem to get good thumb extension so it is good practice to place the indifferent electrode over the motor points of extensor palmaris longus and abductor palmaris longus, about three fingerbreadths proximal to the wrist.
• If thumb extension is still not good, make this electrode the active, assuming this does not significantly reduce finger and wrist extension.
• Care should be taken to avoid either radial or ulna deviation of the wrist. If there is excessive ulnar deviation, move the active electrode towards the extensor carpi radialis brevis on the radial side of the arm. If radial deviation occurs, move the electrode towards the ulna side and the extensor carpi ulnaris.
PICTURE
Thumb Abduction and Opposition
• Radial nerve stimulation can be effective at opening the hand but thumb extension alone can leave the thumb in a less than functional position.
• Abduction and opposition can be produced by stimulating the thenar eminence.
• Place the active electrode over the motor point of Abductor poliicis brevis or opponens pollicis and the indifferent over the back of the wrist. To combine this movement with a general extensor pattern it can be useful to use a ‘Y’ connector.
PICTURE
Reaching
• It is often useful to combine muscles to produce a gross pattern of movement, similar to the combination movements used in every day life. In this way it may be possible to more effectively re-train function rather than by practicing individual muscle activity.
• Reaching is where finger, thumb and wrist extension from radial nerve stimulation are combined with elbow extension and shoulder flexion by all channels on together.
PICTURE
Dosage and Parameters
There are a wide variety of dosage and parameters in the literature. However, the SSAHPF (2014) has been developed to provide guidance for the recommended dosage and parameters for upper limb motor control recovery after stroke. Before selecting ES as a treatment it is important that you are aware of the different dosage and parameter settings and how these will affect the treatment.
A summary of the main dosage and parameters are outlined below:
TABLES
Descriptions of the common parameters reported in literature with recommeneded ranges are synthesised in the table below:
File:ES parameter and dosage Table .pdf
While guidance has been given above in to the dosage and parameters, the clinician has to adjust these to fit the individual. It has been suggested in a review of ES, that it is the adjustment of these parameters which determines the nature of the evoked action potential response and thus impacts on the amount of muscle force generated as well as patient comfort and safety (de Kroon 2002; Ijzerman et al. 2005).
It is important to take into consideration whether the patient has any upper limb tone or spasticity. The dosage and parameters may need to be adjusted for these. Sailsbury (2002) suggested that if spasticity is present then a slower stimulation with a longer ramp time may be beneficial.
Evidence base ACTIVITY
Currently there is a limited amount of evidence to support the use of ES for upper limb motor control. A number of articles have been synthesised and below are their detailed findings.
A systematic review by Vafander et al. (2014), found that the use of ES does not have any significant benefits over conventional treatment. A number of the articles included focused on early intervention after stroke, and concentrated on methods to measure impairments (spasticity, strength and joint motion), not function or activity. These articles showed positive benefits of ES for motor control, however it is unclear if improvements in muscle activity and joint motion can be translated to improvement in motor function. The studies that found no superiority of ES over a conventional treatment tended to be of higher quality and mostly used methods for measuring function or activity instead of impairment. A very limited number of articles concentrated on the use of ES in the later stages of recovery after stroke. Two out of the three studies found positive benefits, however once again these focused on impairments. This systematic review highlighted the need for further research into the effects of ES on upper motor function after both the early and late stages of recovery post stroke.
Although there is a lack of evidence showcasing the benefits for ES as a treatment on it’s own for upper limb motor function, one study has indicated when used in conjunction with other therapeutic techniques there is an improvement in an individuals motor control. Hara (2008) found that individuals that receive motor, proprioceptive, and cognitive inputs through the daily use of ES may demonstrate significantly greater improvements in voluntary movement and functional use of the hand and arm.
Another article by McCabe et al. (2015), suggested that for severely impaired stroke survivors with upper limb dysfunction the use of ES combined with motor learning (5 hours per day partial and whole-task practice of complex tasks) helped to improve coordination and functional task performance. However, when analysing the effectiveness of motor learning and ES, compared to motor learning alone or in conjunction with robotics there were no significant differences.
At this stage it is hard to say whether ES is an effective treatment due to the limited literature. Future research is needed with a greater consistency throughout the studies. More studies need to be undertaken with larger sample sizes, the use of ES in early and late stages of rehabilitation after stroke being explored. Furthermore, the use of standardised outcome measures for function and activity will strengthen the generalisabilty for the use of ES for upper limb recovery post stroke.
KEY POINTS
[edit | edit source]
Dosage and Parameters[edit | edit source]
There are a wide variety of dosage and parameters in the literature. However, the SSAHPF (2014) has been developed to provide guidance for the recommended dosage and parameters for upper limb motor control recovery after stroke. Before selecting ES as a treatment it is important that you are aware of the different dosage and parameter settings and how these will affect the treatment.
In order to understand the recommended dosage and parameters for motor recovery read pages 66-71 from the Scottish Stroke Allied Health Professionals Forum 2014.
The link to this document can be found below:
http://www.chss.org.uk/documents/2014/10/electrical-stimulation-consensus-statement-ssahpf-pdf.pdf
While guidance has been given above in to the dosage and parameters, the clinician has to adjust these to fit the individual. It has been suggested in a review of ES, that it is the adjustment of these parameters which determines the nature of the evoked action potential response and thus impacts on the amount of muscle force generated as well as patient comfort and safety (de Kroon 2002; Ijzerman et al. 2005).
It is important to take into consideration whether the patient has any upper limb tone or spasticity. The dosage and parameters may need to be adjusted for these. Sailsbury (2002) suggested that if spasticity is present then a slower stimulation with a longer ramp time may be beneficial.
A summary of the main dosage and parameters are outlined below:
Frequency
In order to achieve a muscle contraction and minimise patient discomfort and fatigue while maximising clinical benefits has been reported as 12.5Hz (Scheffler and Chae 2007). However, it has also been reported that somewhere between 20-50Hz is appropriate (de Kroon et al. 2005, Sijuth 2008), with lower frequencies required for the upper limb (Scheffler and Chae 2007).
Pulse amplitue and pulse width
To achieve greater muscle force generation through recuitment of neurons increasingly further from the electrode, pulse amplitude and pulse width may (usually 200-400 micro sec) need to be adjusted (Scheffler and Chae 2007, Shu-Shyuan 2002). It has been suggested that the intensity frequency and pulse width of electrical current should be adjusted in order to produce a visible contraction. Although there is agreement in this area, there is still variablity in application and the final decision will fall to the clinician when addressing the individual patient.
Length of treatment
Common doses and duration of treatments delivered range from 30minutes once per day to one hour three times per day for two weeks to three months (de Kroon et. Al. 2005) although this was not substantiated or justified by the original authors. Hsu (2012) randomised 95 participants to dosages of 0, 15, 30, 60 minutes of ES five times per week for four weeks and reported improved recovery in the upper limb with more intensive ES. However, de Kroon et al. (2005) suggested that the particular treatment parameters may not in fact be the critical element in the efficacy of ES within their study so it may be that individual patient treatment approaches may be sufficient.
Most evidence does not justify the choice of ramp times, stimulation wave forms or on/off cycle times so recoomendations regarding these are difficult to make. However, Hsu (2012) reported cycles of 10 seconds on 10 seconds off in the first two weeks and 10 seconds on and 5 seconds off in the second two weeks.
Descriptions of the common parameters reported in literature with recommeneded ranges are synthesised in the table below:
File:ES parameter and dosage Table .pdf
(CHSS Electrical Stimulation Consensus, 2014)
Evidence base[edit | edit source]
Currently there is a limited amount of evidence to support the use of FES for upper limb motor control. A number of articles have been appraised and below are their detailed findings.
A systematic review by Vafander et al. (2014), found that the use of FES does not have any significant benefits over conventional treatment. A number of the articles focused on early intervention after stroke, and concentrated on methods to measure impairments (spasticity, strength and joint motion), not function or activity. These articles showed positive benefits of FES for motor control. However, it is unclear if improvements in muscle activity and joint motion can be translated to improvement in motor function. The studies that found no superiority of FES over a conventional treatment tended to be of a higher quality and mostly used methods for measuring function or activity instead of impairment. A very limited number of articles concentrated on the use of FES in the later stages of recovery after stroke. Two out of the three studies found positive benefits, however once again these focused on impairments. This systematic review highlighted the need for further research into the effects of FES on upper motor function after both the early and late stages of recovery post stroke.
Although there is a lack of evidence showcasing the benefits for FES as a treatment on it’s own for upper limb motor function, one study has indicated when used in conjunction with other therapeutic techniques there is an improvement in an individuals motor control. Hara (2008), found that individuals that receive motor, proprioceptive, and cognitive inputs through the daily use of FES may demonstrate significantly greater improvements in voluntary movement and functional use of the hand and arm.
Another article by McCabe et al. (2015), suggested that for severely impaired stroke survivors with upper limb dysfunction the use of FES combined with motor learning (5 hours per day partial and whole-task practice of complex tasks) helped to improve coordination and functional task performance. However, when analysing the effectiveness of motor learning and FES, compared to motor learning alone or inconjuction with robotics there were no significant differences.
At this stage it is hard to say whether FES is an effective treatment due to the limited literature. Future research is needed with a greater consistency throughout the studies. More studies need to be undertaken with a larger sample size, they also need to explore the use of FES in early and late stage rehabilitation after stroke. Furthermore, the use of standarised outcome measures for function and activity will strengthen the generalisabilty for the use of FES for upper limb recovery post stroke.
The articles used in this section are referenced below if you wish to read these in full:
• HARA, Y., 2008. Neurorehabilitation with New Functional Electrical Stimulation for Hemiparetic Upper Extremity in Stroke Patients. Journal Nippon Medical School. [online]. Vol 75, no 1. [viewed 20 October 2015]. Available from: https://www.jstage.jst.go.jp/article/jnms/75/1/75_1_4/_pdf
• MCCABE, J., MONKIEWICZ, M., HOLCOMB, J., PUNDIK, S., AND DALY, J., 2015. Comparison of Robotics, Functional Elextrical Stimulation, and Motor Learning Methods for Treatment of Persistent Upper Extremity Dysfunction After Stroke: A Randomized Controlled Trial. Archieves of Physical Medicine and Rehabilitation. [online]. Vol 96, pp981-990. [viewed 19 October 2015.] Available from: http://www.archives-pmr.org/article/S0003-9993(14)01228-3/pdf
• VAFADAR, A., COTE, J., AND ARCHAMBAULT, P., 2014. Effectiveness of Functional Electrical Stimulation in Improving Clinical Outcomes in the Upper Arm Following Stroke: A Systematic Review and Meta-Analysis. Biomedical Research International. [online]. [viewed 19 October 2015]. Available from: http://www.hindawi.com/journals/bmri/2015/729768/
Case Study[edit | edit source]
Mrs Jones is a 62 year old women who lives alone she has had a hemiplegic stroke two months ago. Following her Stroke she has found the activities of daily living more difficult. Decreased motor control of her left upper limb is one of Mrs Jones key problems. Mrs Jones also suffers from spasticity in her left arm. As a result she finds dressing and cooking particularly difficult. Mrs Jones was previously very active and wishes to return to higher levels of function. She has been progressing well however recently her progress has been beginning to slow.
Questions:
- Is ES a suitable treatment for the motor recovery of Mrs Jone's left upper limb?(Using relevant evidence to guide your decision making)
- What factors would you have to take into consideration when treating Mrs Jones?
- Where would you apply ES to when you wanted to achieve: 1. elbow extension? 2. reaching?
- What dosage and parameters would you set for each application?
Conclusion[edit | edit source]
Upper limb loss of function is a common consequence of stroke and reported in l significant proportion of those suffering a stroke. Recovery typically is not as good as experienced in the lower limb. Physiotherapists and other health professions can electrical stimulation as part of their treatment regimes. ES for lower limb has been widely adopted however its use for upper limb is less consistently applied.
There is robust evidence and guidelines supporting the use of electrical stimulation to prevent and treat shoulder subluxation, particularly when applied early. This may help with shoulder pain and improve function although the evidence supporting this is less robust and requires greater investigation due to conflicting and poor quality research.
The use of electrical stimulation for recovery of upper limb function and to support motor learning principles is also gaining credibility although currently the evidence based in conflicting and its practice is varied across health boards, however, there is recent promising evidence supporting its benefits with very little advsere side affects (mainly skin irritation), therefore it would be beneficial to consider its use as an adjunct modality, particularly for those who have some arm movement allowing the electrical stimulation to support greater practise.
Selection of parameters guidance has varied widely however recommendations have been suggested which is based on the most robust evidence, predominately extracted from the SSAF consensus statement (SSAF 2014). It should be noted however that appropriate tailoring will be required to achieve the optimum setting for each participant and this requires judgement of the therapist working in conjunction with the patient.
Resources
[edit | edit source]
Bellow are key resources for further information in relation to the use of electrical stimulation for upper limb after stroke.
| NICE | Clinical guideline 162 |
| Royal College of Physicians | National clinical guideline for stroke |
| SIGN | Guideline 118 - management of patients with stroke |
| SSAF | Electrical stimulation - a consensus statement |
Recent Related Research (from Pubmed)[edit | edit source]
Feed goes here!!|charset=UTF-8|short|max=10