Dupuytren’s Contracture
Original Editor - Gwen Fritsche and Lauren Leimbach from Temple University's Evidence Based Practice Project
Top Contributors - Lauren Leimbach, Gwen Fritsche, Kim Jackson, Admin, Lucinda hampton, Rachael Lowe, Chrysolite Jyothi Kommu, Kirenga Bamurange Liliane, Laura Ritchie, Vidya Acharya, Scott A Burns, WikiSysop, Fasuba Ayobami, Cindy John-Chu, Lennert De Henau, Evan Thomas, Khloud Shreif, 127.0.0.1 and Carina Therese Magtibay
Clinically Relevant Anatomy[edit | edit source]
Dupuytren contracture is a progressive disease of the palmar fascia which results in shortening, thickening and fibrosis of the fascia and aponeurosis of the palm.
The palmar fascia is continuous with the antebrachial fascia, the deep fascia of the forearm, and the layer of fascia that covers the dorsum of the hand. The palmar fascia is thicker in the center of the palm and fingers where it forms the palmar aponeurosis and digital sheaths, respectively. The palmar aponeurosis covers the soft tissues of the palm and long flexor tendons. As the longitudinal bands of the palmar aponeurosis undergo fibrosis, the metacarpophalangeal and proximal interphalangeal joints get pulled into flexion. The fourth metacarpal is most commonly affected, followed by the fifth, third, and second. Recently, Dupuytren disease has become a more widely adopted term than Dupuytren contracture to name this condition, as the fingers are not always held in a fixed flexion deformity.[1]
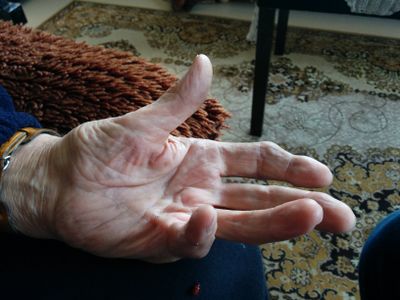
Image 1: Clinical presentation of Dupuytren contracture[2]
Pathological Process[edit | edit source]
The pathophysiology of Dupuytren disease involves abnormal myofibroblastic growth in the hand.
- Type III collagen predominates, which under a nondisease state would be Type I collagen.
- Dupuytren contracture progresses through three phases: (1) proliferative, (2) involution, and (3) residual. The proliferative phase has a characteristically high concentration of immature myofibroblasts and fibroblasts arranged in a whorled pattern. In the involution phase, fibroblasts become aligned in the longitudinal axis of the hand following lines of tension. In the residual phase, relatively acellular collagen-rich chords remain causing contracture deformity.
- The disorder is not always progressive and in at least 50-70% of patients, it may stabilize or even regress.
Several cords can develop which can cause unique deformities of the hand.
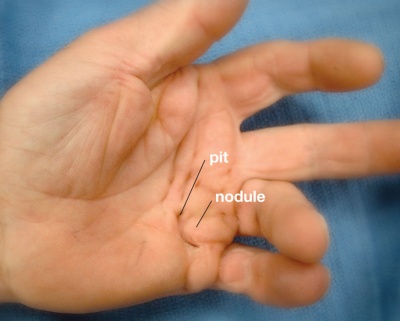
- Pretendinous cords cause skin pitting and metacarpal phalangeal (MCP) joint contracture.
- Natatory cords are responsible for web space contractures.
- Spiral cords are the most important in the disease process and can cause proximal interphalangeal (PIP) contracture.
Risk factors for increased severity and recurrence of disease after treatment include: male gender; onset before age 50; bilateral disease; sibling/parent involvement; presence of Garrod pads, Ledderhose, or Peyronies diseases.[3]
Clinical Presentation[edit | edit source]
Dupuytren contracture occurs slowly and typically progresses over the course of several years, but can also develop more rapidly over weeks or months.[4]
It typically affects older men of European decent. This condition most commonly begins with thickening of the skin on the palm, resulting in a puckering or dimpled appearance. As the condition progresses, bands of fibrotic tissue form in the palmar area and may travel distal toward the fingers. This tightening and shortening eventually leads to the affected fingers being pulled into flexion. Dupuytren contracture typically occurs bilaterally, with one hand being more severely affected than the other.
Physical findings:
- Blanching of the skin when the finger is extended
- Proximal to the nodules, the cords are painless
- Pits and grooves may be present
- The knuckle pads over the PIP joints may be tender
- If the plantar fascia is involved, this indicates more severe disease (Ledderhose disease)
- The patient may not be able to place the palm flat on the table[3]
Diagnostic Procedures[edit | edit source]
- X-rays of the hand should be obtained to examine for other contributing, bony abnormalities that may contribute to the loss of range of motion.
- Laboratory workup to rule out diabetes is recommended.
- Ultrasound may demonstrate thickened palmar fascia and the nodules.[3]
Differential Diagnosis[edit | edit source]
Dupuytren disease should be distinguished from other diseases of the hand including stenosing flexor tenosynovitis, ganglion cysts, and soft tissue masses[3].
Outcome Measures[edit | edit source]
Because flexion contractures of the fingers are one of the primary impairments of Dupuytren disease, range of motion measurements of the metacarpophalangeal (MCP), proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints should be recorded. This can include flexion and extension of these joints, with measurements of passive and active range of motion. These measurements should be taken as a baseline measure and then throughout the treatment process, as they can also be used to help stage the severity of the contractures.
It is also necessary to measure hand function which can be achieved by tests and measures such as the Disabilities of the Arm, Shoulder, and Hand Questionnaire (The DASH), or its shorter version The Quick Dash.
Medical Management[edit | edit source]
| [5] |
Surgical intervention is the current mainstay treatment for Dupuytren disease.[6] Surgical procedures for the correction of flexion contractures in the digits due to Dupuytren disease can be divided into four main categories: simple fasciotomy, partial fasciectomy, total fasciectomy, and dermofasciectomy.[7] These operative procedures are listed in order from least invasive to most invasive.
A simple fasciotomy can be performed either percutaneously or through small incisions and involves the surgeon dividing the contracted tissue cord to release the flexion contracture.[6] During a simple fasciotomy, the contracted cord is simply cut, but is not surgically removed from the digit. A simple fasciotomy is considered suitable for early-stage Dupuytren disease involving passively correctable flexion contracture in the metacarpophalangeal joints alone.
A fasciectomy involves removal of the diseased palmar fascia, including the contracted tissue cord and nodule.[6] A fasciectomy can either be partial or total depending on the severity of the disease. A partial fasciectomy involves removal of the diseased palmar fascia, whereas, a total fasciectomy is more invasive, involving the removal of the entire palmar fascia; both areas affected by disease and areas not affected by disease.[7]
Dermofasciectomy is the most invasive surgical procedure for Dupuytren disease. Dermofasciectomy involves removal of the diseased palmar fascia, the contracted tissue cord and nodule included, and all overlying affected skin and subcutaneous fat.[6] After a dermofasciectomy procedure is performed, a full-thickness skin graft is required to cover the surgical site. In cases of chronic advanced proximal interphalangeal joint contracture, external fixators may be indicated in addition to the dermofasciectomy procedure to keep the contracture from recurring. For severe Dupuytren contractures that keep recurring despite several surgical attempts at correction, amputation of the affected digits may be considered as a last resort.
Since Dupuytren disease is a connective tissue disorder of the hand, contracture recurrence is common even after surgical intervention.[1] However, the rate of recurrence is reduced with the greatest removal of the affected tissues. For example, the rate of recurrence of Dupuytren disease is considerably reduced following a dermofasciectomy, where the affected fascia, subcutaneous fat, and skin is removed, than with a fasciectomy alone; removing only the affected fascia.
Due to the high rate of recurrence of Dupuytren disease, it is important for the surgeon to consider the ‘Dupuytren’s diathesis’ before deciding which surgical procedure would be most effective for the patient.[1] The Dupuytren’s diathesis is a collection of features indicative of an unfavorable prognosis; highlighted by an aggressive disease course and increased risk of disease recurrence.
| [8] |
These features include:
- bilateral involvement
- ethnicity (particularly individuals of northern European descent)
- the presence of ectopic lesions (lesions occurring at sites other than the hands)
- a positive family history of Dupuytren disease (at least one sibling or parent with Dupuytren disease)
- male sex
- age of onset before 50 years of age
Individuals with a positive diathesis may choose to delay surgery for fear of their high risk of contracture recurrence.
Physical Therapy Management[edit | edit source]
Because the primary treatment for Dupuytren disease is surgery, the physical therapist’s main role in the intervention process is postoperatively. Physical therapy after surgery typically includes splinting, exercises, and edema and scar interventions.[9] The primary treatment objective is to maintain the range of motion that was obtained through the surgical removal of the fibrotic tissue. Functional range of motion of the fingers is imperative to many activities of daily living, making its preservation key.
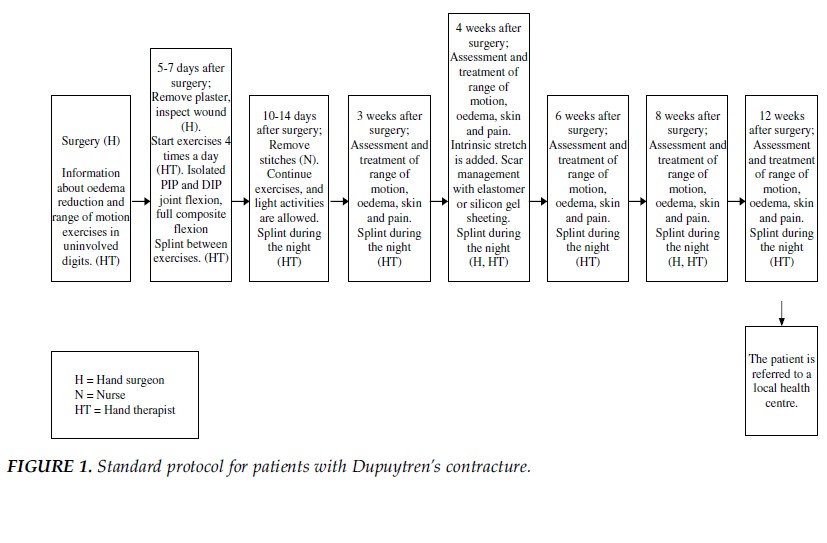
A standard protocol for postoperative management of Dupuytren disease, developed by Engstrand et al. in 2009, is shown in Figure (1).[9] Within the initial 5 days postoperative, the primary interventions are to educate the patient on decreasing edema and the importance of performing range of motion exercises on the uninvolved fingers. After 5-7 days postoperative, the primary interventions shift to range of motion exercises and splinting. The exercises used in Engstrand’s protocol were adapted to each subject’s individual goals and were based on their impairment, physical status, and competency. The types of splints used included volar splints, dynamic extension splint, dynamic flexion splints, exercise splints, and wrist splints.
Image 5: Standard protocol for patients with Dupuytren contracture[9]
While splinting is a widely used intervention for postoperative treatment, Larson and Jarosh-Herold argue its clinical effectiveness, stating that it is only supported by clinical reasoning and anecdotal evidence.[10] According to a systematic review by these authors, the clinical effectiveness of long-term static night splinting on finger movement and hand function remains unproven. They recommend that more research is done using randomized control trials and focus on various types of splints.
Other Interventions[edit | edit source]
Although surgery is considered the mainstay of treatment for Dupuytren disease, not all individuals are willing to undergo surgery.[1] On the other hand, some individuals who elect to have surgery may be deemed unsuitable surgical candidates due to the presence of pre-existing comorbidities, old age, frailty, or lack of social support and assistance needed following surgery. Consequently, numerous nonsurgical treatments have been investigated for Dupuytren disease including hyperbaric oxygen, radiation, ultrasound therapy, vitamin E, physical therapy, steriod injection, interferon, and splinting. However, none of these therapies alone have been proven to be as clinically effective in the management of Dupuytren disease as surgery.
Currently, numerous research studies are demonstrating the clinical safety and effectiveness of Clostridium histolyticum collagenase injection as the promising new nonsurgical treatment for Dupuytren disease.[1] Researchers believe that an abnormal increase in the synthesis and deposition of collagen contributes to the formation of fibrotic tissue and contractures in Dupuytren disease. The clostridial collagenase injection has been shown to be effective in targeting excessive collagen deposition and rupturing the fibrous tissue cords that cause metacarpophalangeal joint and proximal interphalangeal joint contractures in Dupuytren disease.
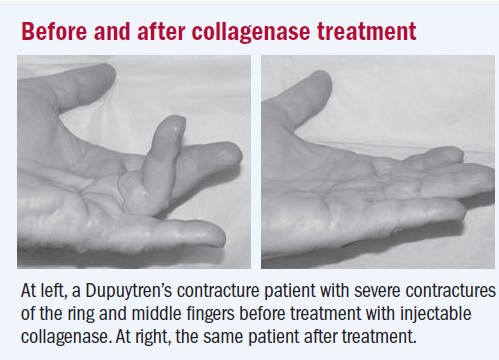
A large prospective, multicenter, phase III clinical trial published in The New England Journal of Medicine in September of 2009, confirmed the effectiveness of Clostridium histolyticum collagenase in decreasing flexion contractures in the digits.[1] In this study, 308 individuals with Dupuytren disease were randomly assigned in a 2:1 ratio to receive up to three injections, 30 days apart, of either 0.58 mg of Clostridium histolyticum collagenase or a placebo injection into the contracted collagen cord. Thirty days after the final injection, 64% of the individuals in the Clostridium histolyticum collagenase injection group displayed a reduction in joint contracture to within 0 to 5 degrees of full extension compared to only 6.8% of individuals in the placebo injection group. However, the percentage of subjects who experienced adverse events was higher in the collagenase injection group with 97% of individuals compared to only 21% of individuals experiencing adverse events in the placebo injection group.[11] Adverse events reported were mostly mild and they included lymph node enlargement and tenderness, bruising, and bleeding, itching, swelling, or pain at the injection site. Of major concern were three serious treatment-related adverse events: two tendon ruptures and one case of complex regional pain syndrome, a progressive disorder causing severe pain, swelling, and skin changes.[11][1] Further studies are needed to determine the long-term benefits and rates of contracture recurrence with the treatment approach of Clostridium histolyticum collagenase injections.[1]
Image 6: Before and after collagenase treatment[11]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Bayat A. A nonsurgical therapy for Dupuytren disease. Rheumatology. 2010;6:7-8.
- ↑ Dupuytren’s Disease. American Society for Surgery of the Hand Web site. http://www.assh.org/Public/HandConditions/Pages/DupuytrensDisease.aspx. 2010. Accessed March 19, 2011.
- ↑ 3.0 3.1 3.2 3.3 Walthall J, Rehman UH. Dupuytrens Contracture. InStatPearls [Internet] 2019 Feb 19. StatPearls Publishing.Available from:https://www.ncbi.nlm.nih.gov/books/NBK526074/ (last accessed 4.4.2020)
- ↑ Mayo Foundation for Education and Research. The Dupuytren's Contracture Page. http://www.mayoclinic.com/health/dupuytrens-contracture/DS00732. Updated May 15, 2010. Accessed March 14, 2011.
- ↑ uwhand. Dupuytren's Contracture Release. Available from: http://www.youtube.com/watch?v=yjYRlo_Zupw[last accessed 25/05/13]
- ↑ 6.0 6.1 6.2 6.3 Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Rheumatology. 2010;6:715-726.
- ↑ 7.0 7.1 Becker GW, Davis TRC. The outcome of surgical treatments for primary Dupuytren’s disease – a systematic review. J Hand Surg Eur. 2010;35E(8):623-626.
- ↑ URehab. Dupuytren's Contracture Treatment Program. Available from: http://www.youtube.com/watch?v=b7niXa484KA[last accessed 25/05/13]
- ↑ 9.0 9.1 9.2 Engstrand C, Boren L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren’s contracture three months after fasciectomy and hand therapy interventions. J Hand Ther. 2009;22:21-27.
- ↑ Larson D, Jerosch-Herold C. Clinical effectiveness of post-operative splinting after surgical release of Dupuytren’s contracture: a systematic review. BMC Musculoskel Dis. 2008;9:104.
- ↑ 11.0 11.1 11.2 Harvard University. Nonsurgical approach unlocks contracted fingers. Harvard Women’s Health Watch. 2009:6-7.