Chronic Pain and the Brain: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
Studies using functional MRI have identified six common regions activated in acute pain. These regions include the primary somatosensory cortex (S1), secondary somatosensory cortex (S2), anterior cingulated cortex, insular cortex, prefrontal cortex and the thalamus. It is these regions that make up the pain neuromatrix which Melzack<ref>Melzack R (2001) Pain and the neuromatrix in the brain. Journal of Dental Education 65(12): 1378-1382</ref> has identified. More information on the Neuromatrix Theory of Pain and the Mature Organism Model can be found on the [[Multidimensional Nature of Pain]] page and on Louis Gifford's website [http://giffordsachesandpains.files.wordpress.com/2013/04/mature-org-ch2-tip1.pdf here].<br> | Studies using functional MRI have identified six common regions activated in acute pain. These regions include the primary somatosensory cortex (S1), secondary somatosensory cortex (S2), anterior cingulated cortex, insular cortex, prefrontal cortex and the thalamus. It is these regions that make up the pain neuromatrix which Melzack<ref>Melzack R (2001) Pain and the neuromatrix in the brain. Journal of Dental Education 65(12): 1378-1382</ref> has identified. More information on the Neuromatrix Theory of Pain and the Mature Organism Model can be found on the [[Multidimensional Nature of Pain]] page and on Louis Gifford's website [http://giffordsachesandpains.files.wordpress.com/2013/04/mature-org-ch2-tip1.pdf here].<br> | ||
In a review conducted by Lithwick et al (2013), the role of these areas in producing pain is explained in depth.<ref>Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45</ref> The primary somatosensory cortex is responsible for sensory discrimination, determining where the pain messaging is coming from. In patients with chronic pain, a more widespread contralateral activation was observed, compared with acute pain where only the ipsilateral side was activated. Another study found that individuals with chronic pain also have a small cortical representation of S1 and implied that “there was dissociation between activation of primary sensory areas and the actual origin of pain.”<ref>Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45</ref> | |||
The secondary somatosensory cortex (S2) is associated with the discrimination of pain intensity and with a coactivation between the primary somatosensory cortex. In patients with chronic pain, there is “a bilateral activation pattern, as compared to a contralateral activation pattern as seen in acute pain.” This indicates that there is less of a representation of the initial pain and may contribute to the widespread, vague pain described by chronic pain patients. | <br> | ||
The secondary somatosensory cortex (S2) is associated with the discrimination of pain intensity and with a coactivation between the primary somatosensory cortex. In patients with chronic pain, there is “a bilateral activation pattern, as compared to a contralateral activation pattern as seen in acute pain.” This indicates that there is less of a representation of the initial pain and may contribute to the widespread, vague pain described by chronic pain patients. | |||
<br>The anterior cingulated cortex (ACC) is associated with emotional and cognitive-evaluative aspects of pain processing along with sensory perceptions due to convergence with the S1. In chronic pain patients, there is bilateral and more widespread ACC activation compared with acute pain.<ref>Maihofner C, Handwerker HO, Birklein F (2006) Functional imaging of allodynia in complex region pain syndrome. Neurology 66(5): 711-717.</ref> The authors concluded that this indicated a heightened sensation of pain in chronic pain patients. Another study found in chronic pain patients “that the ACC and the insula demonstrated a significantly altered correlation to the medial PFC.”<ref>Baliki MN, Baria AT, Apkarian AV. (2011) The cortical rhythms of chronic back pain. Journal of Neuroscience. 31 (39): 13981-13990.</ref> This represents a shift towards a more emotional aspect in chronic pain. | <br>The anterior cingulated cortex (ACC) is associated with emotional and cognitive-evaluative aspects of pain processing along with sensory perceptions due to convergence with the S1. In chronic pain patients, there is bilateral and more widespread ACC activation compared with acute pain.<ref>Maihofner C, Handwerker HO, Birklein F (2006) Functional imaging of allodynia in complex region pain syndrome. Neurology 66(5): 711-717.</ref> The authors concluded that this indicated a heightened sensation of pain in chronic pain patients. Another study found in chronic pain patients “that the ACC and the insula demonstrated a significantly altered correlation to the medial PFC.”<ref>Baliki MN, Baria AT, Apkarian AV. (2011) The cortical rhythms of chronic back pain. Journal of Neuroscience. 31 (39): 13981-13990.</ref> This represents a shift towards a more emotional aspect in chronic pain. | ||
| Line 27: | Line 29: | ||
<br>The prefrontal cortex is responsible for the cognitive evaluation of pain. Three areas are associated with pain: the medial prefrontal cortex (mPFC), dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex. m PFC represents the affective aspect of pain, the DLPFC is involved in localising painful stimuli and the orbitofrontal cortex is a link between painful area discrimination, memory and emotion. Lithwick (2013) observed that in individuals with chronic back pain, m PFC was in a significant hyperactive state, which may reduce brain activity in other areas overall, affecting the brain’s ability to perform other tasks.<ref>Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45</ref> Overall, the change in PFC due to chronic pain represents a shift towards increased emotional processing. | <br>The prefrontal cortex is responsible for the cognitive evaluation of pain. Three areas are associated with pain: the medial prefrontal cortex (mPFC), dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex. m PFC represents the affective aspect of pain, the DLPFC is involved in localising painful stimuli and the orbitofrontal cortex is a link between painful area discrimination, memory and emotion. Lithwick (2013) observed that in individuals with chronic back pain, m PFC was in a significant hyperactive state, which may reduce brain activity in other areas overall, affecting the brain’s ability to perform other tasks.<ref>Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45</ref> Overall, the change in PFC due to chronic pain represents a shift towards increased emotional processing. | ||
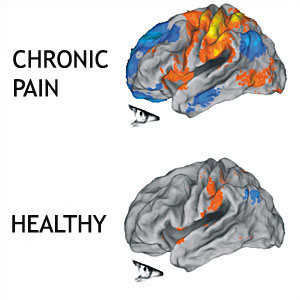
[[Image:Chronic-pain-brain.jpg|frame|center|This image shows that there are many more areas of the brain involved in the perception of chronic pain than acute pain. Credit: Northwestern University.]] | |||
<div><br>Not only are different parts of the brain activated when pain becomes chronic, but there can also be smudging of the sensory and motor homunculus. Smudging refers to the changes in the brain areas devoted to detecting the stimulation of body parts and performing functions begin overlapping. This process is why some body parts may become difficult to use or other areas become sensitive compared to the injured area. For more information on this, please see [[Central sensitisation|central sensitisation]]. Flor (2003) hypothesised that this overall widespread activation and expansion of the representational zone may lead to pain perceptions in the absence of peripheral stimulation.<ref>Flor H. (2003) cortical reorganisation and chronic pain: implications for rehabilitation Journal of Rehabilitative Medicine Suppl. 41: 66-72</ref> </div> | |||
In summary, research has shows that in patients with chronic pain, there is more widespread activation of the areas that are associated with the perception of pain. This can explain the vague, widespread nature of chronic pain. There is also more activation of parts of the brain which are associated with emotional processing in chronic pain states. This may explain some of the emotional changes that can occur with chronic pain states. [[Psychological_basis_of_pain]] offers more information of some of the changes that can occur with chronic pain. | |||
[[Image:Chronic-pain-brain.jpg|frame|center|This image shows that there are many more areas of the brain involved in the perception of chronic pain than acute pain. Credit: Northwestern University.]] | |||
<div><br>Not only are different parts of the brain activated when pain becomes chronic, but there can also be smudging of the sensory and motor homunculus. Smudging refers to the changes in the brain areas devoted to detecting the stimulation of body parts and performing functions begin overlapping. This process is why some body parts may become difficult to use or other areas become sensitive compared to the injured area. For more information on this, please see [[Central sensitisation|central sensitisation]]. Flor (2003) hypothesised that this overall widespread activation and expansion of the representational zone may lead to pain perceptions in the absence of peripheral stimulation.<ref>Flor H. (2003) cortical reorganisation and chronic pain: implications for rehabilitation Journal of Rehabilitative Medicine Suppl. 41: 66-72</ref> </div> | |||
<br>These changes in brain anatomy are a result of increased nociceptive input from the periphery which results in the systemic and anatomical changes discussed above. Wand et al (2011) conclude that “it is likely that part of the pain experience of CLBP patients is mediated by sensitivity changes within the central nervous system and the demonstrated brain changes are a probable contribution to this."<ref>Wand BM, Parkitny L, O’Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley L. (2011) Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual Therapy 16: 15-20</ref> Nociceptive information accesses the cortex through multiple pathways in the spinal cord. Modulating of these cortical areas occurs due to a continuous barrage of nociceptive inputs associated with chronic pain. | <br>These changes in brain anatomy are a result of increased nociceptive input from the periphery which results in the systemic and anatomical changes discussed above. Wand et al (2011) conclude that “it is likely that part of the pain experience of CLBP patients is mediated by sensitivity changes within the central nervous system and the demonstrated brain changes are a probable contribution to this."<ref>Wand BM, Parkitny L, O’Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley L. (2011) Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual Therapy 16: 15-20</ref> Nociceptive information accesses the cortex through multiple pathways in the spinal cord. Modulating of these cortical areas occurs due to a continuous barrage of nociceptive inputs associated with chronic pain. | ||
Revision as of 16:08, 13 January 2015
- Please do not edit unless you are involved in this project, but please come back in the near future to check out new information!!
- If you would like to get involved in this project and earn accreditation for your contributions, please get in touch!
Tips for writing this page:
- Describe the long term impacts of pain on the brain sturcture and it's implications on the pain experince
Original Editor - Add a link to your Physiopedia profile here.
Top Contributors - Kirsten Stower, Kim Jackson, Rishika Babburu, Laura Ritchie, Simisola Ajeyalemi, Rachael Lowe, Jo Etherton, 127.0.0.1, George Prudden, Michelle Lee, Lauren Lopez, Jess Bell and Olajumoke Ogunleye
Brain Areas in the Neuromatrix and the Changes Due to Chronic Pain.[edit | edit source]
Studies using functional MRI have identified six common regions activated in acute pain. These regions include the primary somatosensory cortex (S1), secondary somatosensory cortex (S2), anterior cingulated cortex, insular cortex, prefrontal cortex and the thalamus. It is these regions that make up the pain neuromatrix which Melzack[1] has identified. More information on the Neuromatrix Theory of Pain and the Mature Organism Model can be found on the Multidimensional Nature of Pain page and on Louis Gifford's website here.
In a review conducted by Lithwick et al (2013), the role of these areas in producing pain is explained in depth.[2] The primary somatosensory cortex is responsible for sensory discrimination, determining where the pain messaging is coming from. In patients with chronic pain, a more widespread contralateral activation was observed, compared with acute pain where only the ipsilateral side was activated. Another study found that individuals with chronic pain also have a small cortical representation of S1 and implied that “there was dissociation between activation of primary sensory areas and the actual origin of pain.”[3]
The secondary somatosensory cortex (S2) is associated with the discrimination of pain intensity and with a coactivation between the primary somatosensory cortex. In patients with chronic pain, there is “a bilateral activation pattern, as compared to a contralateral activation pattern as seen in acute pain.” This indicates that there is less of a representation of the initial pain and may contribute to the widespread, vague pain described by chronic pain patients.
The anterior cingulated cortex (ACC) is associated with emotional and cognitive-evaluative aspects of pain processing along with sensory perceptions due to convergence with the S1. In chronic pain patients, there is bilateral and more widespread ACC activation compared with acute pain.[4] The authors concluded that this indicated a heightened sensation of pain in chronic pain patients. Another study found in chronic pain patients “that the ACC and the insula demonstrated a significantly altered correlation to the medial PFC.”[5] This represents a shift towards a more emotional aspect in chronic pain.
The insular cortex (IC) has a role in both sensory and affective perception of pain – the suffering aspect of pain. It is often coactivated with the anterior cingulated cortex. In chronic pain, there is a more widespread activation of the IC compared to acute pain. The IC is also more linked with sensory discrimination in chronic pain, rather than emotional processing. The IC’s role in suffering “ is minimised so as to accommodate the vast increase in activation that is seen in S1 and S2.”[6] This change would contribute to allodynia and hyperalgesia as seen in chronic pain.
The prefrontal cortex is responsible for the cognitive evaluation of pain. Three areas are associated with pain: the medial prefrontal cortex (mPFC), dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex. m PFC represents the affective aspect of pain, the DLPFC is involved in localising painful stimuli and the orbitofrontal cortex is a link between painful area discrimination, memory and emotion. Lithwick (2013) observed that in individuals with chronic back pain, m PFC was in a significant hyperactive state, which may reduce brain activity in other areas overall, affecting the brain’s ability to perform other tasks.[7] Overall, the change in PFC due to chronic pain represents a shift towards increased emotional processing.
In summary, research has shows that in patients with chronic pain, there is more widespread activation of the areas that are associated with the perception of pain. This can explain the vague, widespread nature of chronic pain. There is also more activation of parts of the brain which are associated with emotional processing in chronic pain states. This may explain some of the emotional changes that can occur with chronic pain states. Psychological_basis_of_pain offers more information of some of the changes that can occur with chronic pain.
Not only are different parts of the brain activated when pain becomes chronic, but there can also be smudging of the sensory and motor homunculus. Smudging refers to the changes in the brain areas devoted to detecting the stimulation of body parts and performing functions begin overlapping. This process is why some body parts may become difficult to use or other areas become sensitive compared to the injured area. For more information on this, please see central sensitisation. Flor (2003) hypothesised that this overall widespread activation and expansion of the representational zone may lead to pain perceptions in the absence of peripheral stimulation.[8]
These changes in brain anatomy are a result of increased nociceptive input from the periphery which results in the systemic and anatomical changes discussed above. Wand et al (2011) conclude that “it is likely that part of the pain experience of CLBP patients is mediated by sensitivity changes within the central nervous system and the demonstrated brain changes are a probable contribution to this."[9] Nociceptive information accesses the cortex through multiple pathways in the spinal cord. Modulating of these cortical areas occurs due to a continuous barrage of nociceptive inputs associated with chronic pain.
A study conducted by Seminowicz et al in 2011 investigated the effect of chronic pain on brain anatomy and whether effective treatment would reverse these changes. They found that in chronic pain patients, there was a decreased cortical thickness in the DLPFC, anterior insula, anterior cingulate cortex and primary somatosensory cortex.[10] The loss of cortical thickness corresponds with a loss of neurons and a slower speed of synapses. They then treated the patient’s pain with a nerve root block or spinal surgery to determine if these cortical areas became thicker again with reduced pain. The only statistically significant finding was an increase in the thickness of the DLPFC in patients who reported a decrease in pain intensity and improvements in physical impairments. The authors concluded that more research is needed to determine if interventions targeting psychosocial rather than biomechanical components of chronic pain would result in similar recovery of cortical thickness.
Effects of Chronic Pain on Cognition and Working Memory[edit | edit source]
Working memory refers to the short term, information retention system which is essential to the skill of maintaining and manipulating behaviourally relevant information.[11] Effective working memory function is necessary for guiding behaviour, making decisions and reasoning and planning. Chronic pain patients often display cognitive or working memory deficits and are often impaired in the performance of cognitively demanding tasks.[12]
Legrain et al (2009) developed a neurocognitive model of attention to pain which aims to explain these changes in patients with chronic pain. They explain the modes of attention selection: the bottom-up capture of attention and the top down modulation of attendion. “The bottom-up capture of attention corresponds to an unintentional stimulus-driven capture of attention by events themselves”[13] The involuntary capture of pain is the most important feature of the pain alarm system to interrupt ongoing actions and prioritise behaviours to prevent injury. Legrain et al (2009) reports that studies have found that attention is unintentionally captured if the pain is intense, new and threatening and this is evident in poorer task performance whilst experiencing the pain.
Legrain et al (2009) then explains how the top-down modulation of attention capture can reduce or modulate the pain response. “Numerous studies have shown reduced pain when attention is directed away from nociceptive stimuli.”[14] Neuroimaging studies have shown that responses to painful stimuli in the brain are decreased when attention is strongly focused on a primary visual task. However, the top-down variables may also facilitate attention capture. Attention can be more easily captured by pain if a painful stimulus occurs to a part of the body which is involved in a task, for example, if the pain felt in the hand when the patient has been asked to write. Legrain also found that in patients who expect a stimulus to be painful, there is more attention discruption when a non-painful stimulus occurs. Legrain et al (2009) conclude that in chronic pain, it is likely that there is a tendency to increase attention allocation to pain-related information and less ability to modulate pain with the top-down modulation due to expectations of stimulus being painful.
There have been a number of studies which have tried to understand how an increased attention to pain can then lead to decreased working memory. Walteros et al (2011) compared fifteen patients with fibromyalgia and fifteen healthy patients and looked at how they performed the Iowa Gambling Task and a conditional associative learning task. The results showed that fibromyalgia patients had a poorer performance than healthy controls in both tasks. The patients with fibromyalgia displayed more errors in the learning task and more disadvantageous decisions in the gambling task.[15]
Berryman et al (2013) conducted a systematic review to determine if chronic pain was the cause of the deficits in working memory. They critically appraised twenty four studies, including the study conducted by Walteros et al (2011). They found that in studies that used behavioural outcome measures, chronic pain was associated with decreased verbal working memory, decreased non verbal memory, decreased attention, decreased auditory control and decreased visual memory. However, the results of the EEG and functional MRI studies did not reflect the results of the behavioural studies. There was no difference between chronic pain patients and healthy controls when examining latency or amplitude.[16]
The authors also concluded that there was a high risk of bias due to recruitment, failure to screen for psychiatric disorders, no clear diagnostic criteria for chronic pain and lack of blinding. “Our only empirical evidence of working memory deficits in chronic pain are at high risk of bias which leads us open to the possibility that no such deficits really exist”. They recommend that for future research, studies should use sequential recruitment, apply recognised criteria for chronic pain, screen the control group for chronic pain and both groups for psychiatric disorders, blind both assessors and patients and use larger sample sizes to obtain more valid results.
Implications for Treatment
[edit | edit source]
The changes in the brain that occur due to chronic pain are due to the plasticity of the neural system and the brain. It is this underlying plasticity which suggests that these changes may be responsive to targeted treatment. Moseley and Flor (2012) group these treatments into cognitive-behavioural, sensory and motor strategies.
Moseley and Floor (2012) state that the goals of cognitive-behavioural treatment for chronic pain are to “increase healthy behaviours related to work, leisure time and the family; reduce medication and change the responses of significant others from solicitous to distracting or ignoring.” Patients are taught strategies such as distraction, meditation or relaxation and cognitive reappraisal along side pacing and activity planning to prevent flare ups. They are also educated about pain and the factors which lead to chronic pain and also the factors which can modulate it. Studies have shown that this therapy improves functional capacity and reduces pain, enabling the patient to establish a sense of control over pain and develop behaviours that limit the impact of pain on quality of life.[17]
Wand et al (2011) found that there is increasing evidence that graded motor imagery is effective in treating Complex Regional Pain Syndrome, and Moseley and Flor (2012) also support this. All three authors have also concluded that sensory discrimination training programs have been effective in reducing pain and increasing function in Phantom Limb Pain patients.[18],[19] However, Wand et al (2011) warns of the need to be cautious in generalising this data to the chronic low back pain population, as significant research still needs to be done in this area before firm recommendations can be made about this type of management approach.[20]
References[edit | edit source]
References will automatically be added here, see adding references tutorial.
- ↑ Melzack R (2001) Pain and the neuromatrix in the brain. Journal of Dental Education 65(12): 1378-1382
- ↑ Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45
- ↑ Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45
- ↑ Maihofner C, Handwerker HO, Birklein F (2006) Functional imaging of allodynia in complex region pain syndrome. Neurology 66(5): 711-717.
- ↑ Baliki MN, Baria AT, Apkarian AV. (2011) The cortical rhythms of chronic back pain. Journal of Neuroscience. 31 (39): 13981-13990.
- ↑ Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45
- ↑ Lithwick A, Lev S, Binshtock A (2013) Chronic pain-related remodelling of cerebral cortex – ‘pain memory’: a possible target for treatment of chronic pain. Pain Management 3(1): 35-45
- ↑ Flor H. (2003) cortical reorganisation and chronic pain: implications for rehabilitation Journal of Rehabilitative Medicine Suppl. 41: 66-72
- ↑ Wand BM, Parkitny L, O’Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley L. (2011) Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual Therapy 16: 15-20
- ↑ Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell C, Shir Y, Ouellet JA (2011) Effective Treatment of Chronic Low Back Pain in Humans Reverses Abnormal Brain Anatomy and Function. The Journal of Neuroscience 31(20): 7540-7550
- ↑ Berryman C, Stanton T, Bowering KJ, Tabor A, McFarlane A, Moseley L (2013) Evidence for working memeory deficits in chronic pain: A systematic review. Pain 154: 1181-1196
- ↑ Walteros C, Sanchez-Navarro JP, Munoz MA, Martinez-Selva JM, Chialvo D and Montoya P (2011) Altered associative learning and emotional decision making in fibromyalgia Journal of Psychosomatic Research 70: 294-301.
- ↑ Legrain V, Van Damme S, Eccleston C, Davis KD, Seminowicz DA and Crombez (2009) A neurocognitive model of attention to pain: behavioural and neuroimaging evidence Pain 144: 230-232.
- ↑ Legrain V, Van Damme S, Eccleston C, Davis KD, Seminowicz DA and Crombez (2009) A neurocognitive model of attention to pain: behavioural and neuroimaging evidence Pain 144: 230-232..
- ↑ Walteros C, Sanchez-Navarro JP, Munoz MA, Martinez-Selva JM, Chialvo D and Montoya P (2011) Altered associative learning and emotional decision making in fibromyalgia Journal of Psychosomatic Research 70: 294-301.
- ↑ Berryman C, Stanton T, Bowering KJ, Tabor A, McFarlane A, Moseley L (2013) Evidence for working memeory deficits in chronic pain: A systematic review. Pain 154: 1181-1196
- ↑ Moseley L and Flor H (2012) Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabiliation and Neural repair 26(6): 646-652.
- ↑ Wand BM, Parkitny L, O’Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley L. (2011) Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual Therapy 16: 15-20
- ↑ Moseley L and Flor H (2012) Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabiliation and Neural repair 26(6): 646-652.
- ↑ Wand BM, Parkitny L, O’Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley L. (2011) Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual Therapy 16: 15-20