Lumbar Spinal Stenosis
Original Editors - Stefanie Van De Vijver, Pauline Bouten, Astrid Lahousse, Roxane Roosens and Laure Lievens
Top Contributors - Donald John Auson, Astrid Lahousse, Stefanie Van De Vijver, Roxane Roosens, Kim Jackson, Pauline Bouten, George Prudden, Laure Lievens, Manisha Shrestha, Rosie Swift, Mariam Hashem, Lucinda hampton, Sai Kripa, Shreya Pavaskar and Ahmed M Diab
Definition/Description[edit | edit source]
Lumbar spinal stenosis (LSS)
- is a degenerative condition in which there is diminished space available for the neural and vascular elements in the lumbar spine secondary to degenerative changes in the spinal canal.[2]
- can cause radiating pain and numbness to the buttock, thigh, or leg particularly during walking or standing for a long time. The pain reduces usually when a patient is in resting, sits down, or bends forward.
- is related to aging, affecting mostly individuals over the age of 60 years.[3]
Not all patients with spinal narrowing develop symptoms, so the term "spinal stenosis" refers to the symptoms of pain and not to the narrowing itself and a diagnosis of spinal stenosis is only made once symptoms are present.[4]
Despite its prevalence, currently, there is no universally accepted definition of lumbar spinal stenosis, and there is also a lack of generally accepted radiologic diagnostic criteria. Lumbar spinal stenosis is a significant cause of disability in the elderly, and it is the most significant cause of spinal surgery in patients over 65 years of age. [5]
Clinically Relevant Anatomy[edit | edit source]
Lumbar spinal stenosis refers to an anatomic and pathologic condition that includes the narrowing of the lower spinal canal (central stenosis) or one or more lumbar vertebral foramina (foraminal/lateral stenosis).
There are 33 spinal cord nerve segments in a human spinal cord and 5 lumbar segments that form 5 pairs of lumbar nerves.[7]
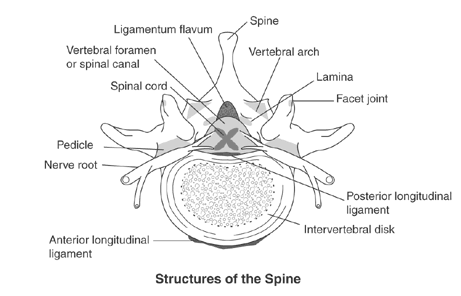
Following structures of the spine are most involved in lumbar spinal stenosis:
- Disc intervertebral (intervertebral discs): There is one disc between each pair of vertebrae, except for the first cervical segment, the atlas. The center is called the nucleus pulposus. This nucleus is surrounded by the annulus fibrosus, which contains several layers of fibrocartilage. the intervertebral disc acts as shock absorbers.
- Facet joints: Synovial joints located on the back of the main part of the vertebra. They are formed by the superior articular process of the underlying vertebra and the inferior articular process of the vertebra lying above it. They connect the vertebrae to each other and permit backward motion.
- Foramen intervertebral (intervertebral foramen): An opening between vertebrae through which nerves leave the spine and extend to other parts of the body.
- Ligaments: Fibrous bands of connective tissue that connect two or more bones together and help stabilize joints. They support the spine by preventing the vertebrae from slipping out of line as the spine moves. A large ligament often involved in spinal stenosis is the ligamentum flavum, which runs as a continuous band from lamina to lamina in the spine.
- Medulla spinalis (Spinal cord/nerve roots): Throughout the entire spine there is a central spinal canal that contains the spinal cord. It is a long, thin, tubular bundle of nervous tissue that is surrounded by the vertebrae. The spinal cord is part of the central nervous system providing a means of communication between the brain and the rest of the body.
- Cauda equina: A sack of nerve roots that continues from the lumbar region, where the spinal cord ends and continues down to provide a neurologic function to the lower part of the body[8]
The spinal canal is the cavity within the vertebral column which contains the spinal cord with its associated nerve roots and vessels.
The spinal canal becomes progressively narrower from its superior opening at the foramen magnum to its inferior opening at the sacral hiatus. The canal itself is primarily formed by the vertebral foramen of adjacent vertebrae. Allowing for variation, the spinal cord occupies the superior two-thirds of the spinal canal and terminates at approximately the middle of the L1 vertebral body.
Boundaries[edit | edit source]
- anterior: vertebral bodies, intervertebral discs, posterior longitudinal ligament
- posterior: ligamentum flavum lining the laminae
- lateral: vertebral pedicles[10]
Epidemiology[edit | edit source]
The prevalence of relative and absolute LSS increased respectively from 16.0% to 38.8% and from 4.0% to 14.3% between age <40 years and 60+ years. If we take a closer look at the 60-69 year-old group, the prevalence of acquired stenosis increases with age to relative 47.2% and absolute 19.4%.[11],[12]
It is an extensive problem in the elderly, presenting with pain, disability, fall risk, and depression.[13]
Spinal stenosis is one of the most common causes of non-traumatic spinal cord injuries[14] in people older than 50 years.[15] Because of the aging of the population, incidence rates of acquired (or degenerative) spinal stenosis have been increasing. This kind of stenosis is due to the degenerative changes (ligamentum flavum, disc intervertebral, and facet joints) related to aging and occurs at the age of 50 and beyond[16].
There are several types of spinal stenosis. Lumbar spinal stenosis and cervical spinal stenosis are the most common types and occur separately or combined. The thoracic spine is rarely involved. Epidemiological data suggests an incidence of 1 case per 100 000 for cervical spine stenosis and 5 cases per 100 000 for lumbar spine stenosis [17]. The incidence of both types increases during the aging process[18][19][20][21][22]
Etiology[edit | edit source]
Some people are born with a small spinal canal. This is called "congenital stenosis”. However, spinal canal narrowing is most often due to age-related changes that take place over time. This condition is called "acquired spinal stenosis." Spinal stenosis is most common in people over 50 years of age.[23]
Acquired forms of LSS are further classified as degenerative, spondylolisthetic, iatrogenic (postsurgical), posttraumatic, or combined.[23]
Lumbar spinal stenosis can be caused by:
- Degenerative spondylosis - With aging, wear-and-tear changes, and traumas, amongst other factors, the intervertebral discs can degenerate and protrude posteriorly, causing increased loading of the posterior elements of the vertebrae. This can lead to posterior vertebral osteophyte formation (uncinate spurs), facet hypertrophy, synovial facet cysts, and ligamentum flavum hypertrophy, which in turn will cause spinal stenosis.
- Degenerative spondylolisthesis - When degenerative changes of the spine occur, the pars interarticularis can be fractured, and the resulting instability can lead to forward translation of the vertebra. Sufficient anterior slippage of one vertebra on top of the next vertebral segment (most commonly L4-on-L5) can narrow the spinal canal, leading to stenosis.
- Other acquired conditions, although rarer than the aforementioned conditions, include space-occupying lesions, post-surgical fibrosis, and rheumatologic conditions as well as other skeletal diseases such as ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis or be secondary to congenital causes such as achondroplasia, which can lead to short pedicles with medially placed facets.[5]
- Osteoarthritis
- Inflammatory spondyloarthritis
- Bulging of the disc
- Thickening of the vertebral ligament
- Tumour
- Infection
- Various metabolic bone disorders that cause bone growth, such as Paget's disease [3][23]
Characteristics/Clinical Presentation[edit | edit source]
- Classically, lumbar spinal stenosis presents as pain exacerbated by prolonged ambulation, standing, and with lumbar extension, and is relieved by forward flexion and rest. Neurogenic claudication is an important feature of lumbar spinal stenosis. The patients complain of pain or discomfort that radiates to the buttock, thigh and lower leg after walking for a certain distance, therefore leading to functional disability and decreased walking capacity.
- Shopping Cart sign
- Symptoms are typically bilateral, but usually asymmetric.
- Low back pain, numbness, and tingling are present in a majority of patients.
- Numbness and tingling in lumbar spinal stenosis involve usually the entire leg and rarely involves only a single nerve root distribution.
- Approximately 43 percent of the patients experience weakness.
- Patients may also report walking upstairs being easier than walking downstairs, as the back is forward flexed with stairs climbing.
- Priaprism (erectile dysfunction) is a rare symptom of lumbar spine stenosis[24]. This presentation is complex and is believed to be a parasympathetic mediated autonomic disorder.
- If patients present with new-onset bowel or bladder dysfunction, saddle anesthesia, bilateral lower extremity weakness and/ or increased lower extremity, the patient may have developed cauda equina or conus medullaris syndromes.
- Not all patients with spinal narrowing develop symptoms, so the term "spinal stenosis" refers to the symptoms of pain and not to the narrowing itself and a diagnosis of spinal stenosis is only made once symptoms are present.
- It is related to aging, affecting mostly individuals over the age of 40-60 years
Narrowing can occur at different sites. Anatomic/radiographic classification can be applied differentiating between:[25][16][26]:
- Central stenosis: narrowing of the spinal canal
- Lateral recess stenosis: narrowing of the lateral recess (area underneath the facet joints)
- Foraminal stenosis: narrowing of the intervertebral foramen
One single or combination of anatomic variation(s) can occur[26].
- In most cases stenosis occurs at the level of facet joints. At this level pathological changes in the disk and facets and hypertrophy of the ligamentum flavum cause the greatest amount of narrowing. At the level of the pedicles, stenosis is rather uncommon and indicates an underlying congenital of developmental stenosis of the bony canal[16].
- Central stenosis can be caused by degeneration of the vertebral disc. This can lead to narrowing of the spinal canal around the cauda equina[27][28] . Symptoms usually involve buttocks and posterior thighs in a non-dermatomal distribution[29].
- Lateral recess stenosis can be related to the lateral recess[27][28]. Symptoms are usually dermatomal because specific nerves are compressed. Patients may have more pain during rest and at night, but have more walking tolerance than patients with central stenosis[29].
- Foraminal stenosis is related to a narrowing of the spinal foramina [28]. It can be the result of a reduced height of the intervertebral space. Foraminal stenosis is also related to age-related degenerative disease of the lumbar discs and/or lumbar facet joints. This increases bone deposition (i.e. osteophytes) due to abnormal redistribution of load-bearing in the lumbar spine. Thickening of the joint capsule, osteoarthritis of the facet joints and cyst formation can also narrow the spinal canal or IV foramen[30].
- Lateral and Foraminal Stenosis can lead to compression of the nerve roots leaving the spinal canal. The L4-L5 segment is most frequently affected by LSS[a], followed by L3-L4[a], L5-S1 and L1-L2[31]. Spinal stenosis can cause compression of the nerve roots of blood vessels, which can be related to the painful symptoms of spinal stenosis[32].
The symptom most commonly attributed to LSS is neurogenic claudication, also referred to as pseudo-claudication.[33] Symptoms of spinal stenosis often start slowly and get worse over time. Pain in the legs may become so severe that walking, even short distances, is unbearable. Frequently, patients must sit or lean forward to temporarily ease pain. [3][34]
Neurogenic claudication refers to leg symptoms encompassing the buttock, groin and anterior thigh, as well as radiation down the posterior part of the leg to the feet. In addition to pain, leg symptoms can include fatigue, heaviness, weakness and/or paraesthesia. Patients with LSS also can report nocturnal leg cramps and neurogenic bladder symptoms. Symptoms can be unilateral or more commonly bilateral and symmetrical. A key feature of neurogenic claudication is its relationship to the patient’s posture where lumbar extension increases and flexion decreases pain. Symptoms progressively worsen when standing or walking and are relieved by sitting.
Relief with sitting in LSS contrasts with most nonspecific low back pain, which is commonly exacerbated by prolonged sitting. Patients with neurogenic claudication report that lying flat is often associated with less relief while lying on the side (permitting lumbar flexion) is more comfortable.[33]
Some patients may report symptoms that are difficult to definitively attribute to LSS. For example, they may only report low back pain (without leg symptoms), which are typical of neurogenic claudication (e.g., characteristic positional nature of symptoms).[33]
Differential Diagnosis[edit | edit source]
During the differential diagnosis, red-flag symptoms must be assessed. If such symptoms are present, further diagnostic workup is immediately warranted.
The differential diagnosis of spinal stenosis is broad and differentiation between several conditions may be complicated because of their frequent coexistence, certainly in the elderly.
Pathologies/diseases that mimic lumbar spinal stenosis are:
- Disc herniation
- Spinal cord primary or secondary tumour
- Peripheral neuropathy
- Osteoarthritis of hips or knees
- Osteoporotic/lumbar compression fracture
- Myofascial pain
- Rheumatoid arthritis
- Lumbar degenerative disk disease
- Lumbar facet arthropathy
- Lumbar spondylosis, spondylolysis, spondylolisthesis and spondylodiscitis
- Mechanical low back pain
- Cauda equina syndrome = red flag
- Peripheral vascular disease (vascular claudication)
- Nonspecific low back pain
- Infection
- Radiculopathy
- Spinal cord vascular malformations
- Tethered cord syndrome.
- Trochanteric Bursitis
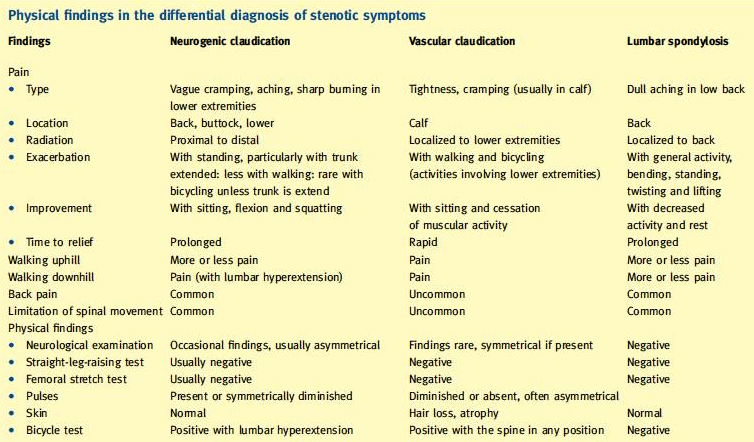
A difference must be made between neurogenic claudication caused by LSS, lumbar spondylosis, or vascular claudication. You can find all the differences between Lumbar spondylosis, neurogenic and vascular claudication in table 1.
Table 1: Physical finding in the differential diagnosis of stenotic symptoms[33]
Older patients (>60-65 years) with back or leg pain, diagnostic possibilities differ from younger patients: non-mechanical causes of back pain, such as malignancy, infection or abdominal aortic aneurysm are common in elderly patients. [7]
Diagnostic Procedures[edit | edit source]
Diagnosis is made by a doctor based on patient history and physical examination. In addition, medical imaging can be performed to confirm the diagnosis. First the clinical diagnosis of LSS, and exclusion of other competing diagnoses: the history and medical history of the patient should be questioned. Ask for a description of symptoms and for any injury, condition, or general health problem that might be causing the symptoms.
The therapist checks for pain or symptoms when the patient hyper-extends the spine (bends backwards), and checks for normal neurologic function (for instance, sensation, muscle strength, and reflexes) in the arms and legs.
Furthermore, a variety of measurements can be used to assess treatment of patients with LSS. The underlying cause of LSS is identified by imaging techniques such as[11]
- Radiography is usually the first step to identify a degenerative process (disc degeneration, osteophytes, facet hypertrophy). It is also helpful in the evaluation of the alignment, loss of disc height and osteophyte formation. With oblique views a defect of the pars interarticularis is detectable while instability is detectable with dynamic views. Instability is confirmed when the view shows a translation of more than 5 mm or a rotation of more than 10-15 degrees. Reversal of the normal trapezoidal disc geometry with widening posteriorly and a narrowing anteriorly can also indicate instability. The diagnosis of spinal stenosis can be made when bony narrowing, obliteration of epidural fat and deformities of the spine is detected on the X-ray[29].Some other signs of LSS include narrowing height of intervertebral foramina and space of intervertebral disc, small interlaminar window, hypertrophy of facet joint, short pedicles, thick lamina, and deep posterior concavity of vertebral bodies[35]
- MRI (Magnetic Resonance Imaging) (T2 weighted) is used for determining the degree of stenosis and the thickness of Ligamentum Flavum. MRI is very sensitive to degeneration and is used for the evaluation of lateral recess stenosis. Sagittal T2-images focus on the foramen vertebrale and are used to diagnose central stenosis. A lack of fat around the root indicates foraminal stenosis. The most common ones for central LSS are anteroposterior canal diameter, cross sectional area; for lateral LSS, the most common ones are the height and depth of the lateral recess and the lateral recess angle; for foraminal stenosis, they are foraminal diameter and height, hypertrophic facet joint degeneration and foraminal nerve root impingement.[5] Axial T1-images are used to search for extraforaminal stenosis by showing an obliteration of the normal interval of fat between the disc and the nerve root. MRI findings should always be matched to the symptoms and signs of patients with neurogenic claudication of radiculopathy[29]. In many cases MRI replaces CT-scans[29]. Degenerative changes of the spine are visible on MRI and increase with age. These degenerative changes appear in nearly 100% of people over the age of 60. 21% of people over the age of 60 are diagnosed with spinal stenosis. The sensitivity of MRI is 87-96% and its specificity is 68-75%. Preferably an MRI is taken with the patient in upright position. This enables the visualization of the patho-anatomical changes which occur during axial loading [36].
- Diameter of 10 mm of the spinal canal equals absolute stenosis;
- Diameter of 12 mm indicates severe stenosis.
- By means of a CT scan a cross-section of the spinal canal may reveal the effects of disk pathology, facet hypertrophy and buckled Ligamentum Flavum. Nonetheless a CT scan has poor soft-tissue contrast, which is why a myelography is usually conducted simultaneously. The combination of both displays a good image of the center lateral canal and defines any extradural cause of compression. This diagnostic procedure is preferred in case of dynamic stenosis, when there are contraindications for MRI or in the absence of suitable findings on MRI. Direct measurements of the bony canal on CT-scans should be interpreted with caution, because they often give an inaccurate assessment of the degree of stenosis[29].
- Triple sequence[13]
- Ultrasound (US) [13]
- Myelography[13]
- Bone scan (shows where bone is breaking down or being formed)[37].
To date, we are unaware of an identified association between patient report of symptoms, functional outcomes (Oswestry), Visual Analog Scale (VAS), and anatomical impairment in patients with LSS.[13]
The severity of the structural pathology has a poor correlate with the severity of symptoms and limitation.[23]
In fact, examination of asymptomatic subjects showed that >30% had canal narrowing that would be classified as consistent with LSS. Imaging therefore cannot be considered a gold-standard in diagnosis of LSS, and must only be considered as an adjunct to a thorough physical examination.[13]
Outcome Measures[edit | edit source]
Lumbar spinal stenosis is related to neurogenic claudication, which is a leading cause of pain and disability. Therefore, specific questionnaires can be used for the assessment of a patient with lumbar spinal stenosis (LSS)[38][39].
- The Swiss Spinal Stenosis Questionnaire also known as The Zurich Claudication Questionnaire (ZCQ) consists of three subscales that measure symptom intensity, satisfaction with treatment and physical function. The physical function scale is used primarily to evaluate walking capacity, which is reduced in patients with lumbar spinal stenosis[32][40][41][42]. This questionnaire is a valid and reliable instrument for assessing patients with lumbar spinal stenosis[32][39][42].
- The Oswestry Disability Index for assessing self-reported levels of disability and The Maine-Seattle Back Questionnaire (a modification of the Roland-Morris Disability Questionnaire) are commonly used for patients with LSS. They are also valid and reliable instruments for that population.[39][43][42].
- The Modified Oswestry Disability Index (MOSW).[44][45][46]
- The Satisfaction Subscale of the Spinal Stenosis Scale (SSS); this is a test for psychological well-being.
- A Numeric Pain Rating Scale (NRPS) for average thigh/leg pain [47]
- The Oxford Spinal Stenosis Score[44]
- Visual analogue scale [45][46]
- Roland-Morris Disability Questionnaire[46]
- A self-administered, self-reported history questionnaire (SSHQ)[48]
- Other frequently used questionnaires to determine the progression of symptoms, such as pain and other outcomes, such as level of satisfaction or disability are the Patient Specific Functional Scale, and the Short Form 36 health survey (SF-36).[32][39][42][49][50].
Examination[edit | edit source]
The physical examination for patients with LSS is usually normal or demonstrates nonspecific findings. Patients with stenosis often have lumbar, paraspinal, or gluteal tenderness, which is usually related to underlying degenerative changes, muscle spasms, and poor posture.
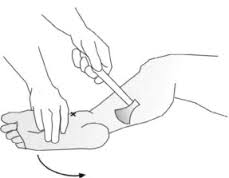
The neurologic examination is usually normal or reveals only subtle abnormalities such as mild weakness, sensory changes, and reflex abnormalities. The achilles tendon reflexes (Fig. 3) are often diminished, while abnormal knee reflexes are less common. The straight leg-raise test and other neural tension signs are usually negative unless there is accompanying disc herniation. Hamstring tightness is often present and may produce a false-positive straight leg-raise test.[51][3]
Possible symptoms that occur during the examination are neurogenic claudication, which includes pain in the buttocks, thigh or leg during ambulation that improves during rest, or radicular leg symptoms with associated neurological deficits. These symptoms have to present themselves for at least 12 weeks. An older person with suspicion of spinal stenosis usually stoops forward while walking. Other possible symptoms should always be considered during the examination, including rare symptoms such as pripism[24]. Pedal pulses, pain on hip rotation and other tests should be performed depending on which symptoms occur and a full neurological examination should be done[52][37].
Following tests should be conducted:
Bicycle Stress Test
During this test the patient first pedals on a cycle ergometer in upright position with preservation of neutral lumbar lordosis. The distance the patient has pedaled in a certain amount of time is recorded. The patient has to pedal a second time in a slumped position with lumbar delordosing. The distance the patient has pedaled in the same time is recorded again. If the patient can pedal further in slumped position than in upright position, lumbar spinal stenosis is indicated[37][52].
Upright posture with lumbar lordosis Slumped posture with decreased lumbar lordosis
Two-Stage Treadmill Test
This test is evaluated on a treadmill. When the patient walks on the flat (0°) treadmill their back is in an extended position. The walking distance in a certain amount of time is recorded. The patient walks on the treadmill a second time with an uphill slope, which means they walk in a flexed position. The walking distance in the same amount of time is recorded again. If the patient walks further on an uphill slope than on the flat treadmill, lumbar spinal stenosis is indicated[37][52].
Flat treadmill Uphill slope
Exercise stress testing on a treadmill - The study of Deen et al [53] determined that this test procedure is a save and easy way to asses baseline functional status and surgical outcome in patients with neurogenic claudication due to lumbar spinal stenosis. By comparing pre- and postoperative results they concluded that this test procedure provides objective outcomes. Thus, this study claims that surgery is beneficial in most cases and is helpful in guiding subsequent management of patients with residual symptoms. The latter is interesting for the physical examination in patients with spinal stenosis.
The test is performed on a treadmill at 0° ramp incline. There are two trials conducted for 15 minutes. The first time the patient should walk at a speed of 1,2 miles per hour and the second time the patient can walk at a pace of their own choosing. Between those two efforts there is a brief rest permitted to allow symptoms to return to baseline level.
The researchers have created a division in degrees on the basis of the occurrence of symptoms and their severity:
- Grade 1 = patients who were able to walk symptom-free
- Grade 2 = patients who were able to complete the test with some neurologic symptoms
- Grade 3 = patients who were able to walk 5 – 15 minutes
- Grade 4 = patients who were able to walk less than 5 minutes
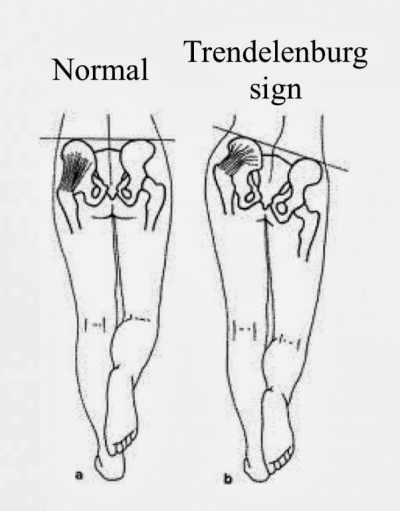
A careful motor examination should be performed. Leg weakness is generally mild and in the distribution of the L4, L5, or S1 nerve roots. Weakness of the muscles innervated by L5 is the most common finding. If the physiotherapist wants to demonstrate the spinal level truly responsible for the symptoms of LSS, it is recommended to use the gait-loading test which is a provocation test . The lumbar extension-loading test (Fig. 4) is useful for assessment of lumbar spinal stenosis pathology and is capable of accurately determining the involved spinal level. In this test, you have to maintain the lumbar region in moderate (angle of 10°-30°)extension while standing as long as you can. After the patient has performed this test, changes in subjective symptoms and objective neurological findings can be evaluated[54]. The examiner should test for weakness of great toe extensors and hip abductors as well. The Trendelenburg test (Fig. 5) is used to observe for hip abductor weakness. Difficulty with walking on the toes suggests S1 root involvement. Difficulty with heel walking suggests L4 or L5 nerve dysfunction.[3]
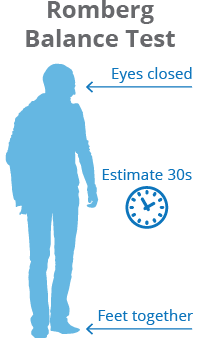
Katz et al report physical examination findings most strongly associated with lumbar spinal stenosis (LSS) include wide-based gait, abnormal Romberg test, (Fig. 6) thigh pain following 30 seconds of lumbar extension, and neuromuscular abnormalities[51]; however, Fritz et al state physical examination findings do not seem helpful in determining the presence or absence of LSS.[55]
The classic presentation of LSS is radiating leg pain associated with walking that is relieved by rest (neurogenic claudication). When patients bend forward, the pain diminishes.
Physical examination findings are frequently normal in patients with LSS. Nevertheless, review of the literature suggests diminished lumbar extension appears most consistently, varies less, and constitutes the most significant finding in LSS. Other positive findings include loss of lumbar lordosis and forward-flexed gait.[56]
Fig. 3: Achilles tendon reflexes
Fig. 4: Lumbar extension loading test
Fig. 5: Trendelenbrug sign Fig. 6: Romberg balance test
Medical Management[edit | edit source]
Many therapeutic modalities could be used in the management of spinal stenosis. Treatment plans must be individualized based on each specific patient's presentation. Spinal stenosis rarely leads to progressive neurological injury. Therefor non-operative modalities should be attempted first.
Management for lumbar spinal stenosis is aimed at reducing symptoms and improving functional status.
Conservative treatment is the first-line treatment for this condition.
Conservative treatment options include physical therapy, oral anti-inflammatory medications, and epidural steroid injections. Although there is no standardized physical therapy regimen, many therapists focus on stretching and strengthening of the core muscles, which can lead to correction of posture and improved symptom.
Although there are short-term benefits, lumbar epidural steroid injections have not been shown to have long-term improvement of pain and disability in lumbar spinal stenosis patients, and there is no statistical difference between epidural injections with anesthetics alone versus a mixture of anesthetics with corticosteroids.
Lumbar corsets may also be trialed for temporary relief of pain[5]
Medication[edit | edit source]
Steroid Injections[edit | edit source]
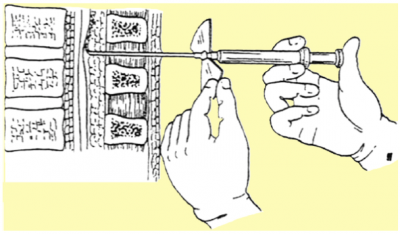
Nerve roots may become irritated and swollen at the area where they are pinched. Injecting a corticosteroid into the space around the compression can help reduce the inflammation and relieve some of the pressure. It is suggested that epidural steroid injections help to control severe radicular symptoms in patients with spinal stenosis. However, repeated steroid injections can weaken nearby bones and connective tissue.[3]
fig. 7: Infusion method of the anaesthetic into the epidural space to treat the radicular symptoms is illustrated.[33]
Non-steroidal Anti-Inflammatory Medications[edit | edit source]
Non-steroidal anti-inflammatory medications (NSAIDs) are commonly prescribed for patients with LSS, and often help relieve pain associated with spinal stenosis. By reducing inflammation, these medications can relieve some of the pressure on compressed nerves.[57]
Epidural injections with steroids or with local anesthetic alone provide significant pain relief and functional improvement in managing chronic low back pain secondary to lumbar spinal stenosis, and the inclusion of steroids confers no advantage compared to local anesthetic alone.[58]
Surgical Management[edit | edit source]
The primary goal of surgery is to decompress the affected roots. According to the individual differences in severity, chronicity and the location, the appropriate type of decompression should be selected. No one procedure can solve all types of stenosis. The principle of surgery is to achieve neural decompression. It is easy to underestimate the extent of the decompression required.[33]
Over time, surgical treatment has become progressively less invasive.
The following prognostic indicators help to provide the surgeon with realistic expectations of the results of decompression so that the patient can be counselled appropriately. [33]
Barring emergencies such as cauda equina syndrome, surgical management for lumbar spinal stenosis is usually elective, as the goal of treatment is to improve function, rather than preventing neurologic impairment.
The decompression approaches include conventional laminectomy, bilateral laminotomy, unilateral laminotomy with contralateral recess decompressed by transmedia way, partial facetectomy and split-spinous process laminotomy/laminoplasty.[5]The most frequently performed surgical procedure is laminectomy. A randomized trial has shown that there is a greater improvement of symptoms in patients undergoing laminectomy compared to the non-surgical group, however, the symptom improvement between the surgical and non-surgical groups diminish over time.
Laminectomy with fusion is typically reserved for patients with concurrent spondylolisthesis to provide further stability.
A less invasive approach is interspinous spacer implantation, this procedure is appropriate for patients with intermittent claudication symptoms without spondylolisthesis.[5]
Good prognostic factors include:
- Adequate decompression is achieved,
- Facet joint stability is maintained.
- Early decompression surgery is performed,
- Postoperative corset is worn and exercises can be performed.
Poor prognostic factors include:
- Persistence of back pain as a predominating symptom,
- Multi-level decompression,
- Prolonged delay to surgery after onset of symptoms, and
- Preoperative presence of significant neurological deficits including urinary symptoms
Operative Techniques[edit | edit source]
Decompression by Interspinous Process Distraction Devices This procedure is best indicated for extension dynamic stenosis. The patient should have evidence of relief of symptoms with flexion and worsening of symptoms with extension.[33][edit | edit source]
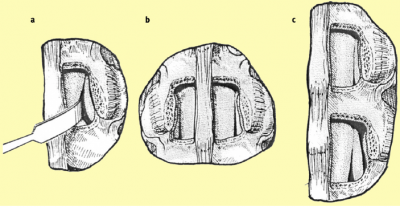
Decompression The ultimate surgical goal is adequate decompression of the neural structures, including both the cauda equina and exiting nerve roots (Figures 8 and 9).[33] 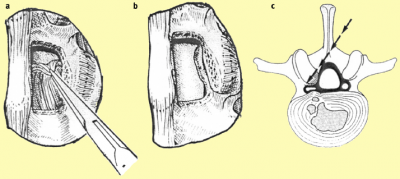 [edit | edit source]
[edit | edit source]
a. After removing the ligamentum flavum bony overgrowth is visualized and can be removed carefully
b. After removal of the medial third of the inferior facet laterally, the superior facet osteophyte can be seen intruding into the lateral recess of the spinal cana
c. The osteophyte can be removed using an osteotome or sharp curette
a. The nerve root previously trapped deep to the superior facet osteophyte will become visible following removal of this osteophyte.
b. The selective decompression can be repeated on the other side of the canal preserving the important midline ligamento-osseous to maintain stability structures
c. The level above can be decompressed if necessary
Physical Therapy Management[edit | edit source]
LSS patients frequently receive early surgical treatment, although conservative treatment can be a viable option. Not only because of the complications that can arise from surgery, but also because mild symptoms of radicular pain often can be lightened with physical therapy.[59]
Its specific content and effectiveness relative to other nonsurgical strategies has not been clarified yet.[60] Postoperative care after spinal surgery is variable, with major differences reported between surgeons in the type and intensity of rehabilitation provided and in restrictions imposed and advice offered to participants. Postoperative management may include education, rehabilitation, exercise, behavioral graded training, neuromuscular training and stabilization training.[61]
Low quality evidence suggests an improvement in walking ability wearing a lumbar corset in comparison with an elastic woolen corset or no corset.[62] It can be worn during daily activities that require walking, but only worn for only a limited number of hours each day, otherwise the paraspinal muscles can atrophy[11]
Furthermore, low quality evidence indicates that modalities such as ultrasound, TENS, heat packs and manual therapy in addition to an exercise program, do not upgrade the active exercises.[62]
In general, two recent level 1A reviews on literature[60][62] concluded that there was no consistent evidence for the effectiveness of physical exercise therapy. The specific elements of physical therapy that may benefit should further be examined, as well as the optimal dosage. Cycling and other exercises performed during flexion of the spine, are usually better tolerated than walking.[34]
Lee et al.[29] found that patients with moderate pain undergoing a conservative treatment will have 50% pain relief in less than 3 months. In total 60–90% of all patients experience symptom relief after surgical or conservative treatment.
Physical therapy is associated with reduced likelihood of patients receiving surgery within 1 year. Only symptomatic patients should be treated[63].
There are no significant differences between conservative treatment and surgery for pain up to 2-year follow-up. From then on the results of the study favour surgery over physical therapy for pain and disability[41]. Malmivaara et al favour surgery over physical therapy for all time points. Weinstein et al gave no differences between groups[41].
The study of Atlas et al.[63] concluded that at the end of the follow up period there is no difference in pain and overall satisfaction between patients who were treated with physical therapy or with surgery. Radicular symptoms did improve less after physical therapy.
Conservative treatment and especially physical therapy includes a combination of different interventions:
- Bed rest[63]
- Flexion-based exercise programs [41][63][64] -Lumbar flexion exercises are done to reduce the lumbar lordosis. This is the most comfortable position for the patient because the symptoms reduce in combination with a decrease of the epidural pressure in the lumbar spinal canal. -Single and double leg knees to chest in supine position. This position should be held maintained for 30 seconds. In the single leg exercise the patient should alternate the legs. Double knee is a progressive exercise. -This exercise program should have a stepwise logistic regression during the first 6 weeks. -Treadmill walking is the final step in this program.
- Manual therapy[41]
- Lumbar isometric and stretching exercises[41]
- Static and dynamic postural exercises [41]
- Individualized muscle strengthening [41]
- In the over 70 age bracket a graded rehabilitation approach focusing on improving ambulation showed significant pain reduction[65]. See the reference for comprehensive outline of rehabilitation exercises.
- Endurance exercises [41]
- Stabilization of abdominal and back muscles to avoid excessive lumbar extension [41]
- Postural and ergonomic advice
- Falls prevention intervention in seniors with LSS. Seniors with chronic low back pain have a significantly higher risk of falls[66]
- Aerobic fitness [29]
- Cycling exercises[41]
- Home exercises[63]
- Education (Back school) and counselling [41][63]
- An aquatic walking and jogging program has a beneficial effect on muscle function tests, the BERG balance scale and the fall efficacy scale. The ankle range of motion also increased. This program still has to be investigated[67].
- Corsets may help to maintain a posture of slight lumbar flexion to avoid atrophy of paraspinal muscles but it should be worn only for a limited number of hours per day. It also promotes back extension. A lumbar corset is significantly better than no corset for pain and walking capacities[41].
The youtube below shows some good exercises to use in treating clients with LSS.
Patient Education[edit | edit source]
Patient education is inherent to physical therapy practice and valued by those with clinical experience in the treatment of patients with LSS.
The patient education package comprise:
- The intent of the manual therapy and exercise interventions
- The course of physical therapy
- The purpose of the home exercise program (HEP)
- Self-management strategies
- Pain sciences information
- Prognosis
An anatomical explanation for the patients symptoms may contribute to a fear avoidance of activity and an over medicalization of the problem[69]
Helpful advice may include items such as:
- Temporary avoidance of prolonged overhead activities
- Temporary avoidance of prolonged axial loading (standing, use of backpacks, prolonged overhead working postures),
- Methods of self lumbo-pelvic flexion and/or rotational stretching techniques for pain control in standing, sitting, and lying.
Basic body mechanics are taught to the patient with LSS, and they should be advised:
- To change positions frequently,
- Know and respect their current limits,
- To pace activities such as housework and yard work[13][70]
Finally, patients should be aware of the natural course of this condition and patients should know that the majority of those with LSS do quite well over time, their condition either remaining the same or improving over time with no intervention at all[71] and that long term results are often no different when comparing those who received surgery for LSS and those who were treated non-surgically[13][72][73][74]
Manual Therapy[edit | edit source]
A recent systematic review[75] concluded that the use of manual therapy in conjunction with exercise is of potential benefit for the LSS population. In a randomized controlled trial[76] they explained that a utilization of manual therapy in a management program is associated with improvements in pain and disability.
Successful results were reported with following techniques:
- Flexion–distraction manipulations,
- Sidelying lumbar rotation thrust,
- Posterior-to-anterior mobilizations,
- Sidelying translatoric side bending manipulations,
- Thoracic thrusts,
- Neural mobilizations[13][72][73][74][77][78]
Manual Therapy Interventions Provided in Rct by Whitman et al[edit | edit source]
The combined use of manual therapy appears to be an effective intervention. Manual therapy appropriately used involves the lumbar region and thoracic region, pelvis, hips and lower extremities.
The manual therapy approach is impairment-based on thrust and non-thrust mobilization/manipulations to the lumbar and pelvic regions. These interventions frequently emphasize rotation, flexion and distraction, but may involve other techniques dependent upon the individual patient presentation.
Normalization of hip motion appears to be a key element for the successful treatment of patients with LSS. Distraction manipulation of the hip is a valuable intervention technique for restoration of hip motion and function[79]
Aerobic Training and Exercise Intervention[edit | edit source]
Evidenced based guideline from the North American Spine Society concluded through work group consensus that treatment by a physical therapist and exercise may be beneficial for those with LSS and neurogenic claudication[80]
Exercise prescription based on expert consensus is done with the purposes of providing improved:
- Overall fitness and function (gains in mobility and strength),
- An adjunct to manual therapy techniques
- Increased available cross-sectional area of the spinal canal
- Vascular changes (Helping in treatment of any concomitant peripheral arterial disease)
- Self-management (decreasing fear avoidance issues related to walking).
- Improved pain modulation through stimulation of large motor pathways.
The hemodynamic changes that occur with movement provide a theoretical basis for the positive, symptom-reducing effects of exercise[81]
Individualized exercises specific to patients with LSS often include components of:
- Unweighted walking or cycling
- Spinal mobility and lumbar flexion exercises
- Hip mobility exercises
- Hip strengthening
- And core strengthening
Unweighted Walking or Cycling[edit | edit source]
Unweighted treadmill walking has been part of a physical therapy plan of care in several studies for patients with LSS[76][82][83]
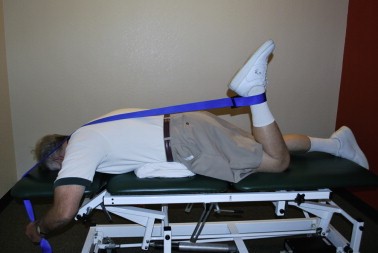
Patients are unweighted to the extent that pain is relieved in order that they can ambulate with good quality movement and pain-free for 30 min[82]. The amount of unweighting is lessened over time as per the patient's response. Fig. 11 shows a patient on the unweighted treadmill. For those who do have unweighting systems available in their clinics, our experience is that patients will have a fairly dramatic response to the unweighting within the first couple of sessions of using the equipment for their aerobic exercise.
If a patient does not have a substantial positive response within the first couple of sessions, we recommend cycling, walking on an inclined treadmill, or other forms of “unloading” such as pool walking.[13]
In an RCT demonstrated that cycling was just as effective as unweighted treadmill walking; therefore cycling is a viable alternative for use as an in-clinic exercise for those without unweighting equipment.[46] 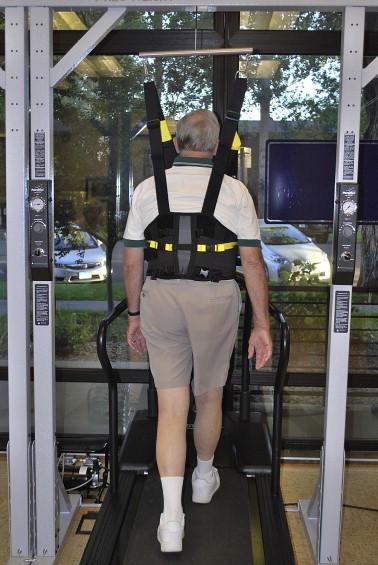
Fig. 10. Unweighted treadmill walking.
Spinal Mobility and Lumbar Flexion Exercises[edit | edit source]
Improved flexibility is frequently a key to intervention, as the presentation of the patient with LSS is often one of overall stiffness. Flexibility impairments can be addressed through manual therapy and self-stretching taught as a home exercise program.
Several case studies and one RCT have used flexion exercises successfully in the treatment of LSS[23][82][76][84]
Other spinal flexibility exercises typically given to the patient include:
- Thoracic extension
- Self-mobilization
- Stretching exercises
- And lumbar rotation exercises.
Patients often experience immediate relief of lower extremity symptoms with the sidelying lumbar rotation exercise.
One of the first exercises taught to patients are therapeutic interventions targeted at maximizing thoracic extension. Those are important because, more flexibility in this region should lessen the extension range of motion required of the lumbar spine during standing and with walking. Extension, without concomitant lumbar extension is frequently necessary for pain free ambulation[82][13][70][83][76]
Hip Mobility Exercises[edit | edit source]
Other muscles around the hip, such as: the hamstrings, rectus femoris, piriformis and tensor fascia latae can become shortened and the patient may respond positively to manual and self-stretching of these muscles.[13]
Hip Strengthening[edit | edit source]
Weakness in the hip extensors and abductors complete the picture of typical muscle imbalances in the hip region of the patient with LSS, and should be addressed through a progressive resistive exercise program that is vigorous enough to affect strength change[82][13]
Core Strengthening[edit | edit source]
Core strengthening/stabilization is a mainstay of most treatment programs for LBP. Therefore, most patients with LSS are appropriate for some level of core strengthening, especially those with impaired strength or motor control of the abdominal and lumbar musculature. It is expected that most core strengthening will be done with a flexion bias and will attempt to allow the patient to control pelvic position and motion to minimize symptoms while standing and walking[82][13][70][83][47]
Specifically, patients can be taught to temporarily use a PPT to relieve symptoms, or even to maintain a slight PPT to lessen or avoid symptoms entirely while standing and/or walking.
Specific evidence directing dosage of exercise prescription is lacking in the patient population with LSS.
Exercise is essential to the treatment of the patient with LSS. Therapists must have a wide range of exercises available as patients with LSS present with a wide range of functional levels and frailty.
Whitman et al. [85] found that a program of spine manipulation, the pelvis and lower extremities, muscle strengthening exercises and body-weight supported treadmill walking had superior outcomes compared to a program that included lumbar flexion exercises, non-thermal ultrasound to the lumbar area, and a treadmill walking program in treating patients with spinal stenosis. The study also concludes that additional improvements in disability, satisfaction and treadmill walking tests can be realized by the inclusion of manual physical therapy interventions, exercise and a progressive body weight supported treadmill walking program[85]. They also found that manual therapy in addition to flexion exercises and walking had similar effects to those of flexion exercises and walking alone[85].
The study of Pua et al.[86]showed that body-weight supported treatment was not significantly better than cycling for short term pain and disability.
Koc et al.[87]found that the addition of modalities (eg.ultrasound, TENS) to the conservative treatment are effective in reducing pain and improving function in patients with LSS. Goren et al. [86] found that an exercise program plus modalities was significantly better than no exercise in short term for back pain, leg pain and disability.
Fritz et al. [63] says that if the recommended initial management strategies are not effective, aggressive conservative interventions should be pursued. This study also confirms that patients who take physical therapy treatment in the first six weeks are less likely to undergo surgery at one year follow up. They found higher levels of patient, who had physical therapy during 6 weeks, self-rated major improvements at 3 – 6 months and 1 year. They also had a greater reduction in leg pain after 1 year[63].
Clinical Bottom Line[edit | edit source]
Lumbar spinal stenosis is a condition where the spinal canal (central stenosis) or one or more of the lumbar vertebral foramina (foraminal/lateral stenosis) becomes narrowed. If the narrowing is substantial, it can cause compression of the spinal cord or spinal nerves. Symptoms of spinal stenosis include low back pain, buttock pain, leg pain and numbness. These symptoms are typically aggravated by walking and relieved by resting. Interventions that may help relieve symptoms of spinal stenosis and prevent progression of the condition include: patient education, manual therapy, aerobic training and excercise intervention (strenghtening, stretching, mobilization, pelvic tilts and lower back stabilization). If non-operative treatment does not relieve symptoms, surgical treatment may be appropriate. Decompressive posterior laminectomy is the most common type of surgery.
Lumbar spinal stenosis is a common disease process. These patients have symptoms such as leg pain and low back pain. The cause of this condition is most likely secondary to chronic wear-and-tear damages to the vertebral column. Because there is no evidence to demonstrate the superiority of surgical versus non-surgical treatments of lumbar spinal stenosis, it is important to take a interprofessional team-based cooperative model. Primary physicians must recognize the patient's presentation, the care of the patient must be coordinated with pain management, orthopedist, and/ or neurosurgeons (if there is a neurosurgical emergency) and physical therapists.
Physical therapy is the mainstay in the conservative treatment of this condition.
References[edit | edit source]
- ↑ Bhave A. Modern Techniques in Spine Surgery. JP Medical Ltd; 2014 Nov 30.
- ↑ Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC, Reitman CA. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). The Spine Journal. 2013 Jul 1;13(7):734-43.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Mazanec DJ, Podichetty VK, Hsia A. Lumbar canal stenosis: start with nonsurgical therapy. Cleveland Clinic journal of medicine. 2002 Nov 1;69(11):909-17.
- ↑ Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handbook of clinical neurology. 2014 Jan 1;119:541-9.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Wu L, Cruz R. Lumbar spinal stenosis. InStatPearls [Internet] 2018 Oct 27. StatPearls Publishing. Available from: Wu L, Cruz R. Lumbar spinal stenosis. InStatPearls [Internet] 2018 Oct 27. StatPearls Publishing.Last accessed 26.1.2020)
- ↑ Depuy Synthes Companies. What is Spinal Stenosis. Available from: https://www.youtube.com/watch?v=31RuBxzXhr0 (last accessed 8.3.2019)
- ↑ 7.0 7.1 Cohen MS, Wall EJ, Kerber CW, Abitbol JJ, Garfin SR. The anatomy of the cauda equina on CT scans and MRI. The Journal of bone and joint surgery. British volume. 1991 May;73(3):381-4.
- ↑ David G. Borenstein, James S. Panagis, Peter C. Gerszten, and James N. Weinstein, Questions and answers about spinal stenosis, National institute of health
- ↑ http://www.niams.nih.gov/Health_Info/Spinal_Stenosis/#spine_c
- ↑ Radiopedia Spinal Canal Available from:https://radiopaedia.org/articles/spinal-canal (last accessed 26.1.2020)
- ↑ 11.0 11.1 11.2 Costandi S, Chopko B, Mekhail M, Dews T, Mekhail N. Lumbar spinal stenosis: therapeutic options review. Pain Practice. 2015 Jan;15(1):68-81.
- ↑ Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: the Framingham Study. The Spine Journal. 2009 Jul 1;9(7):545-50.
- ↑ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 13.12 13.13 Backstrom KM, Whitman JM, Flynn TW. Lumbar spinal stenosis-diagnosis and management of the aging spine. Manual therapy. 2011 Aug 1;16(4):308-17.
- ↑ 5. Ho CH, Wuermser LA, Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC. Spinal Cord Injury Medicine. 1. Epidemiology and Classification. Arch Phys Med Rehabil. 2007 Mar;88(3 Suppl 1):S49-54.[LoE: 2C]
- ↑ Netter FH. Atlas of Human Anatomy. 4th Edition. Philadelphia: Elsevier; 2006.
- ↑ 16.0 16.1 16.2 Skinner HB, McMahon PJ. Current Diagnosis& Treatment in Orthopedics. 5th Edition. U.S.A.: Mc Graw Hill Education; 2014.
- ↑ Melancia JL, Francisco AF, Antunes JL. Handbook of Clinical Neurology: Spinal Stenosis. Handb Clin Neurol. 2014;119:541-9. [LoE: 3A] [Abstract
- ↑ Meyer F, Börm W, Thomé C. Degenerative Cervical Spinal Stenosis. Dtsch Arztebl int. 2008 mey; 105(20): 366-372. [LoE: 3A]
- ↑ Genevay S, Atlas SJ. Lumbar Spinal Stenosis. Best Pract Res Clin Rheumatol. 2010 Apr;24(2):253-65. [LoE: 3A]
- ↑ Wise C., Spinal stenosis, American College of Rheumatology, 2013.
- ↑ Ogiela D., Spinal stenosis, National Library of Medecin, 2012.
- ↑ Cluett J., M.D, Spinal stenosis, Orthopedics, 2010.
- ↑ 23.0 23.1 23.2 23.3 23.4 Fritz JM, Erhard RE, Vignovic M. A nonsurgical treatment approach for patients with lumbar spinal stenosis. Physical therapy. 1997 Sep 1;77(9):962-73.
- ↑ 24.0 24.1 Barbaro K, Midgley J. Priapism, a symptom of claudication of the cauda equina in spinal stenosis. Musculoskeletal Science and Practice. 2021 Apr 1;52:102337.
- ↑ Gallucci M, Puglielli E, Splendiani A, Pistoia F, Spacca G. Degenerative Disorders of the spine. Eur Radiol. 2005 Mar;15(3):591-8. [LoE: 2C]
- ↑ 26.0 26.1 De Graaf I, Prak A, Bierma-Zeinstra S, Thoma S, Peul W, Koes B. Diagnosis of lumbar spinal stenosis: a systematic review of the accuracy of diagnostic tests. Spine. 2006 May;31(10):1168–1176. [LoE: 3A]
- ↑ 27.0 27.1 Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, Aydogan M, Hamzaoglu A. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008 May;17(5):679–85. [LoE: 2C]
- ↑ 28.0 28.1 28.2 Karantanas AH, Zibis AH, Papaliaga M, Georgiou E, Rousogiannis S. Dimensions of the lumbar spinal canal: variations and correlations with somatometric parameters using CT. Eur Radiol. 1998;8(9):1581-85. [LoE: 2B]
- ↑ 29.0 29.1 29.2 29.3 29.4 29.5 29.6 29.7 Lee SY, Kim TH, Oh JK, Lee SJ, Park MS. Lumbar Stenosis: A Recent Update by Review of Literature. Asian Spine J. 2015:9(5):818-828. [LoE: 3A
- ↑ Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008 Feb 21;358(8):818-25. [LoE: 3A]
- ↑ Siebert E, Prüss H, Klingebiel R, Failli V, Einhäupl KM, Schwab JM. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Neurology 2009 Jul; 5(7):392-403. [LoE: 1A]
- ↑ 32.0 32.1 32.2 32.3 Pratt RK, Fairbank JC, Virr A. The Reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the Assessment of Patients With Lumbar Spinal Stenosis. Spine. 2002 Jan 1;27(1):84–91. [LoE: 2C]
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 33.6 33.7 33.8 Moon MS, Kim SS, Sihn JC. Lumbar spinal stenosis–a current view. Orthopaedics and Trauma. 2014 Dec 1;28(6):396-408.
- ↑ 34.0 34.1 Katz JN, Harris MB. Lumbar spinal stenosis. New England Journal of Medicine. 2008 Feb 21;358(8):818-25.
- ↑ Wu AM, Zou F, Cao Y, Xia DD, He W, Zhu B, Chen D, Ni WF, Wang XY, Kwan KY. Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment. AME Medical Journal. 2017.
- ↑ 1. Kalff R, Ewald C, Waschke A, Gobisch L, Hopf C. Degenerative lumbar spinal stenosis in older people – current treatment options. Dtsch Arzetbl Int 2013;110(37):613–24. [LoE: 1A]
- ↑ 37.0 37.1 37.2 37.3 1. Goldman L, et al. Questions and answers about spinal stenosis. National Institute of Arthritis and Musculoskeletal and Skin Diseases. Cecil Medicine. 24th ed. Philadelphia, Pa.: Saunders Elsevier; 2012.
- ↑ Pratt RK, Fairbank JC, Virr A. The Reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the Assessment of Patients With Lumbar Spinal Stenosis. Spine. 2002 Jan 1;27(1):84–91.
- ↑ 39.0 39.1 39.2 39.3 1. Ammendolia C et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev. 2013 Aug;30(8). [LoE: 1A]
- ↑ 1. Tomkins CC et al. Construct validity of the physical function scale of the Swiss Spinal Stenosis Questionnaire for the measurement of walking capacity. Spine. 2007 Aug;32(17):1896-1901. [LoE: 2B]
- ↑ 41.00 41.01 41.02 41.03 41.04 41.05 41.06 41.07 41.08 41.09 41.10 41.11 41.12 1. Macedo LG et al. Physical therapy interventions for degenerative lumbar spinal stenosis: a systematic review. Phys Ther. 2013 Dec;93(12):1646-60. [LoE: 2A]
- ↑ 42.0 42.1 42.2 42.3 1. Cleland JA et al. Psychometric properties of selected tests in patients with lumbar spinal stenosis. Spine J. 2012 Oct;12(10):921-931. [LoE: 2B]
- ↑ 1. Tomkins CC et al. Construct validity of the physical function scale of the Swiss Spinal Stenosis Questionnaire for the measurement of walking capacity. Spine. 2007 Aug;32(17):1896-1901. [LoE: 2B]
- ↑ 44.0 44.1 Pratt RK, Fairbank JC, Virr A. The reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the assessment of patients with lumbar spinal stenosis. Spine. 2002 Jan 1;27(1):84-91.
- ↑ 45.0 45.1 Sinikallio S, Aalto T, Airaksinen O, Herno A, Kröger H, Savolainen S, Turunen V, Viinamäki H. Lumbar spinal stenosis patients are satisfied with short-term results of surgery–younger age, symptom severity, disability and depression decrease satisfaction. Disability and rehabilitation. 2007 Jan 1;29(7):537-44.
- ↑ 46.0 46.1 46.2 46.3 Pua YH, Cai CC, Lim KC. Treadmill walking with body weight support is no more effective than cycling when added to an exercise program for lumbar spinal stenosis: a randomised controlled trial. Australian journal of physiotherapy. 2007 Jan 1;53(2):83-9.
- ↑ 47.0 47.1 Whitman JM, Flynn TW, Childs JD, Wainner RS, Gill HE, Ryder MG, Garber MB, Bennett AC, Fritz JM. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: a randomized clinical trial. Spine. 2006 Oct 15;31(22):2541-9.
- ↑ Konno SI, Kikuchi SI, Tanaka Y, Yamazaki K, Shimada YI, Takei H, Yokoyama T, Okada M, Kokubun SI. A diagnostic support tool for lumbar spinal stenosis: a self-administered, self-reported history questionnaire. BMC musculoskeletal disorders. 2007 Dec 1;8(1):102.
- ↑ Thornes E et al. Prognosis of surgical treatment for degenerative lumbar spinal stenosis: a prospective cohort study of clinical outcomes and health-related quality of life across gender and age groups. Open Orthop J. 2011;5:372-78. [LoE: 2B]
- ↑ 1. Zanoli G et al. SF-36 scores in degenerative lumbar spine disorders: analysis of prospective data from 451 patients. Acta Orthop. 2006 Ap;77(2):298-306. [LoE: 2B]
- ↑ 51.0 51.1 Katz JN, Dalgas M, Stucki G, Katz NP, Bayley J, Fossel AH, Chang LC, Lipson SJ. Degenerative lumbar spinal stenosis Diagnostic value of the history and physical examination. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1995 Sep;38(9):1236-41.
- ↑ 52.0 52.1 52.2 1. Kalff R, Ewald C, Waschke A, Gobisch L, Hopf C. Degenerative lumbar spinal stenosis in older people – current treatment options. Dtsch Arzetbl Int 2013;110(37):613–24. [LoE: 1A]
- ↑ 1. Whitman JM, Flynn TW, Fritz JM. Nonsurgical management of patients with lumbar spinal stenosis: a literature review and a case series of three patients managed with physical therapy. Phys Med Rehabil Clin N Am. 2003 Feb;14(1):77-101. [LoE: 4]
- ↑ Takahashi N, Kikuchi SI, Yabuki S, Otani K, Konno SI. Diagnostic value of the lumbar extension-loading test in patients with lumbar spinal stenosis: a cross-sectional study. BMC musculoskeletal disorders. 2014 Dec;15(1):259.
- ↑ Fritz JM, Delitto A, Welch WC, Erhard RE. Lumbar spinal stenosis: a review of current concepts in evaluation, management, and outcome measurements. Archives of physical medicine and rehabilitation. 1998 Jun 1;79(6):700-8.
- ↑ Hsiang JK, Kishner S. Spinal Stenosis Clinical Presentation. Medscape, Updated Jul. 2015;9.
- ↑ Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A. Surgical versus nonsurgical therapy for lumbar spinal stenosis. New England Journal of Medicine. 2008 Feb 21;358(8):794-810.
- ↑ Meng H, Fei Q, Wang B, Yang Y, Li D, Li J, Su N. Epidural injections with or without steroids in managing chronic low back pain secondary to lumbar spinal stenosis: a meta-analysis of 13 randomized controlled trials. Drug design, development and therapy. 2015;9:4657.
- ↑ Minamide A, Yoshida M, Maio K. The natural clinical course of lumbar spinal stenosis: a longitudinal cohort study over a minimum of 10 years. Journal of Orthopaedic Science. 2013 Sep 1;18(5):693-8.
- ↑ 60.0 60.1 May S, Comer C. Is surgery more effective than non-surgical treatment for spinal stenosis, and which non-surgical treatment is more effective? A systematic review. Physiotherapy. 2013 Mar 1;99(1):12-20.
- ↑ McGregor AH, Probyn K, Cro S, Doré CJ, Burton AK, Balagué F, Pincus T, Fairbank J. Rehabilitation following surgery for lumbar spinal stenosis: a Cochrane review. Spine. 2014 Jun 1;39(13):1044-54.
- ↑ 62.0 62.1 62.2 Macedo LG, Hum A, Kuleba L, Mo J, Truong L, Yeung M, Battié MC. Physical therapy interventions for degenerative lumbar spinal stenosis: a systematic review. Physical therapy. 2013 Dec 1;93(12):1646-60.
- ↑ 63.0 63.1 63.2 63.3 63.4 63.5 63.6 63.7 1. Fritz JM et al. Associations between physical therapy and long-term outcomes for individuals with lumbar spinal stenosis in the SPORT study. The spine journal. 2014;14:1611-21. [LoE: 2C]
- ↑ Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. Bmj. 2016 Jan 4;352. [1]
- ↑ Hoffman H, Bennett SS, Li CH, Haakana P, Lu DC. Minimally Invasive Decompression and Physiotherapy for Lumbar Spinal Stenosis in Geriatric Patients. Cureus. 2018 Jun;10(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6089476/ (last accessed 8.3.2019)
- ↑ Wong AY, Karppinen J, Samartzis D. Low back pain in older adults: risk factors, management options and future directions. Scoliosis and spinal disorders. 2017 Dec;12(1):14. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5395891/ (last accessed 8.3.2019)
- ↑ Lee JH, Sung E. The effects of aquatic walking and jogging program on physical function and fal efficacy in patients with degenerative lumbar spinal stenosis. Journal of Exercise Rehabilitation. 2015;11(5):272–75. [LoE: 2B]
- ↑ Physical Therapy Video. Top 7 exercises to stop Lumbar stenosis pain. Available from: https://www.youtube.com/watch?v=V4YDcYS3dyo (last accessed 8.3.2019)
- ↑ Breslau J, Seidenwurm D. Socioeconomic aspects of spinal imaging: impact of radiological diagnosis on lumbar spine-related disability. Topics in Magnetic Resonance Imaging. 2000 Aug 1;11(4):218-23.
- ↑ 70.0 70.1 70.2 Vo AN, Kamen LB, Shih VC, Bitar AA, Stitik TP, Kaplan RJ. Rehabilitation of orthopedic and rheumatologic disorders. 5. Lumbar spinal stenosis. Archives of physical medicine and rehabilitation. 2005 Mar 1;86:69-76.
- ↑ Johnsson KE, Uden A, Rosén IN. The effect of decompression on the natural course of spinal stenosis. A comparison of surgically treated and untreated patients. Spine. 1991 Jun;16(6):615-9.
- ↑ 72.0 72.1 Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, Singer DE. The Maine Lumbar Spine Study, Part III: 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine. 1996 Aug 1;21(15):1787-94.
- ↑ 73.0 73.1 Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine. 2000 Mar 1;25(5):556-62.
- ↑ 74.0 74.1 Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the maine lumbar spine study. Spine. 2005 Apr 15;30(8):936-43.
- ↑ Reiman MP, Harris JY, Cleland JA. Manual therapy interventions for patients with lumbar spinal stenosis: a systematic review. InDatabase of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet] 2009. Centre for Reviews and Dissemination (UK).
- ↑ 76.0 76.1 76.2 76.3 Whitman JM, Flynn TW, Childs JD, Wainner RS, Gill HE, Ryder MG, Garber MB, Bennett AC, Fritz JM. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: a randomized clinical trial. Spine. 2006 Oct 15;31(22):2541-9.
- ↑ Snow GJ. Chiropractic management of a patient with lumbar spinal stenosis. Journal of manipulative and physiological therapeutics. 2001 May 1;24(4):300-4.
- ↑ DuPriest CM. Nonoperative management of lumbar spinal stenosis. Journal of Manipulative and Physiological Therapeutics. 1993;16(6):411-4.
- ↑ Hoeksma HL, Dekker J, Ronday HK, Heering A, Van Der Lubbe N, Vel C, Breedveld FC, Van Den Ende CH. Comparison of manual therapy and exercise therapy in osteoarthritis of the hip: a randomized clinical trial. Arthritis Care & Research: Official Journal of the American College of Rheumatology. 2004 Oct 15;51(5):722-9.
- ↑ Watters III WC, Baisden J, Gilbert TJ, Kreiner S, Resnick DK, Bono CM, Ghiselli G, Heggeness MH, Mazanec DJ, O'Neill C, Reitman CA. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. The spine journal. 2008 Mar 1;8(2):305-10.
- ↑ Takahashi K, Miyazaki T, Takino T, Matsui T, Tomita K. Epidural pressure measurements. Relationship between epidural pressure and posture in patients with lumbar spinal stenosis. Spine. 1995 Mar;20(6):650-3.
- ↑ 82.0 82.1 82.2 82.3 82.4 82.5 Fritz JM, Erhard RE, Delitto A, Welch WC, Nowakowski PE. Preliminary results of the use of a two-stage treadmill test as a clinical diagnostic tool in the differential diagnosis of lumbar spinal stenosis. Journal of spinal disorders. 1997 Oct;10(5):410-6.
- ↑ 83.0 83.1 83.2 Whitman JM, Flynn TW, Fritz JM. Nonsurgical management of patients with lumbar spinal stenosis: a literature review and a case series of three patients managed with physical therapy. Physical Medicine and Rehabilitation Clinics. 2003 Feb 1;14(1):77-101.
- ↑ Creighton DS, Krauss J, Marcoux B. Management of lumbar spinal stenosis through the use of translatoric manipulation and lumbar flexion exercises: a case series. Journal of Manual & Manipulative Therapy. 2006 Jan 1;14(1):1E-0E.
- ↑ 85.0 85.1 85.2 1. Whitman JM et al. A Comparison Between Two Physical Therapy Treatment Programs for Patients With Lumbar Spinal Stenosis. SPINE. 2006;31(22):2541-49. [LoE: 1B]
- ↑ 86.0 86.1 1. Macedo LG et al. Physical therapy interventions for degenerative lumbar spinal stenosis: a systematic review. Phys Ther. 2013 Dec;93(12):1646-60. [LoE: 2A]
- ↑ Koc Z et al. Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine. 2009 May;34(10):985-89. [LoE: 1B]
![[1]](/images/1/1e/LSS1.jpg)