Rhabdomyolysis: Difference between revisions
m (Text replace - '- Your name will be added here if you are a lead editor on this page.' to ' ') |
m (Text replace - ''''Lead Editors'''' to ''''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}}') |
||
| Line 2: | Line 2: | ||
'''Original Editors '''- [[User:Emily Kordik|Emily Kordik]] from [[Pathophysiology of Complex Patient Problems|Bellarmine University's Pathophysiology of Complex Patient Problems project.]] | '''Original Editors '''- [[User:Emily Kordik|Emily Kordik]] from [[Pathophysiology of Complex Patient Problems|Bellarmine University's Pathophysiology of Complex Patient Problems project.]] | ||
''' | '''Top Contributors''' - {{Special:Contributors/{{FULLPAGENAME}}}} | ||
</div> | </div> | ||
== Definition/Description<br> == | == Definition/Description<br> == | ||
Revision as of 16:54, 14 June 2013
Original Editors - Emily Kordik from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Emily Kordik, Michael Beavin, George Tate, Elaine Lonnemann, Admin, Lucinda hampton, Scott Buxton, Kim Jackson, Jason Copelin, 127.0.0.1, WikiSysop, Candace Goh and Lauren Lopez
Definition/Description
[edit | edit source]
Rhabdomyolysis is the breakdown of skeletal muscle tissue that occurs quickly due to a large release of creatinine phosphokinase enzymes due to mechanical, physical, or chemical traumatic injuries.[1] Due to the quick breakdown of the skeletal muscle there is a big accumulation of the breakdown products which can cause renal failure.[1]
Historical Background[edit | edit source]
The first known report of rhabdomyolysis occurred in Sicily in 1908 after an earthquake, this was also the first case of crush syndrome as well and was found in German military literature.[2][3] [4] While this was the first report there has been some speculation that there are references in the Bible about rhabdomyolysis during the Jews exodus from Egypt. It was described as a plague that occurred after a large intake of quail.[2] [4] A similar incident occurred in 1930 in the Baltic sea area where there was a large consumption of intoxicated fish.[3]
Military</span>
The focus of rhabdomyolysis really came about during World War II, especially during the bombing that occurred in London, where crush victims developed acute renal failure.[2][3] Reports were also present during the Korean War as well as during Vietnam. During Vietnam the incidence actually decreased which is thought to be due to the faster evacuation techniques and improved fluid resuscitation to victims.[2]</span>
Natural Disasters
</span>
As seen through history the most common incidence of rhabdomyolyisis occurs during natural disasters where there are less resources available to helped trapped victims, making their time under rubble longer increasing their chances of developing rhabdomyolysis. On August 17, 1999 in Marmara, a region of Turkey, an earthquake with a 7.4 magnitude devastated the area. This earthquake caused 17,480 deaths. Many victims were sent to hospitals, 9,843 patients were hospitalized with 425 of them dying. Of those 9,843 patients, 639 patients developed renal failure, this was 12% of the patients that were hospitalized. The victims average time spent under rubble was 11.7 hours. [2]
Collapse of World Trade Center
On September 11, 2001 in New York City the twin towers collapsed trapping many victims under rubble. Hospitals were prepared to have dialysis ready the days following the attacks to treat the many victims to prevent renal failure. Fortunately very few victims had crush injuries and only victim developed rhabdomyolysis, a 38 year-old police officer who had been trapped under rubble for 24 hours.[2]
Prevalence[edit | edit source]
Eighty-five percent of victims of traumatic injuries develop rhabdomyolysis.[2] Of those patients with rhabdomyolysis 10-50% of those patients will develop acute renal failure.[2] It is also suggested that victims of severe injury that develop rhabdomyolysis and later acute renal failure have a mortality of 20%.[2] Approximately 26,000 cases of rhabdomyolysis are reported annually in the US.[5] Men have a slightly higher incidence of developing rhabdomyolysis than women.[6]
Pathophysiology[edit | edit source]
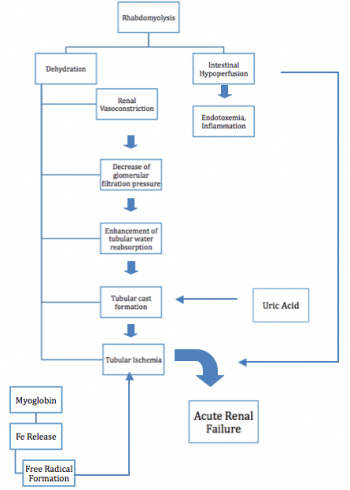
Rhabdomyolysis occurs due to injury whether it is mechanical, chemical, toxins, poisons, or burns, these injuries have a detrimental effect to the cell membranes throughout the body, When a cell membrane is damaged the breakdown or lysis releases organic and inorganic intracellular components such as potassium, myoglobin, lactic acid, purines, and phosphate which enter the circulation. Exhaustive work of cells and stretching can increase sarcoplasmic influx of sodium, chloride, and water, which can result in swelling and autodestruction.[4] After the restoration of blood flow after the injury these components become toxic to the body and in most cases are life threatening, making rhabdomyolysis a medical emergency. [3] "Myoglobin levels rise within hours of muscle damage, but can return to normal in 1-6 hours if continuous muscle injury is not present."[6]
Myoglobin is usually filtered through glomeruli and reabsorbed in the proximal tubules by endocytosis, however when rhabdomyolysis occurs there is an excess of myoglobin, which overloads the proximal tubule cells ability to convert iron to ferritin, which then results in intracellular ferruhemate accumulation.[6] Since iron is able to donate and except electrons as well as having the ability to generate free radicals the urine’s pH can lead to metabolic acidosis. This process puts oxidative stress and injury to the renal cells, which if untreated can lead to renal cell failure.[3]
When there is an excess of myoglobin the tubules are unable to reabsorb it.[3][4] Systemic vasoconstriction sets in which results in water reabsorption in renal tubules, which then increases myoglobin concentration in urine. This in turn causes formation of casts that obstruct renal tubules, another contributing factor of cast formation is apoptosis that occurs in epithelial cells.[6] This obstruction causes formation of free radicals from iron, which can lead to renal failure.[3]
Potassium is another byproduct of muscle lysis. If there is too much potassium in the circulation then hyperkalemia can occur which is life threatening, because of its cardiotoxicty effects, this is a medical emergency.[3] Cardiac arrhythmias can occur due to increased levels of potassium in the blood, in some cases early death occurs due to ventricular fibrillation.[7]
Calcium accumulation in the muscles occurs in the early stages of rhabdomyolysis. Massive calcification of necrotic muscles can occur which can lead to hypercalcemia.[6] If hyperkalemia is present hypercalcemia can lead to cardiac arrhythmias, muscular contraction, or seizures.[4]
Causes[edit | edit source]
Causes for rhabdomyolysis can be broken down into 2 categories, hereditary causes and acquired causes. The most common causes of rhabdomyolysis are prolonged exertion during exercise, trauma, or alcohol abuse. Patients that have been hospitalized are five times more likely to admitted due to non-traumatic causes, than traumatic causes.[6]
Hereditary Causes[edit | edit source]
Those that are at risk for rhabdomyolysis have a family history of disorders dealing with carbohydrate metabolism as well as disorders of lipid metabolism. Disorders of lipid metabolism include malignant hyperthermia, mitochondrial disorders, as well as other genetic disorders.[8]
Acquired Causes[edit | edit source]
Some of the most common acquired causes include trauma or crush injury, toxic, sever muscle exertion, seizures, shaking chills, delerium tremors, ischemia or muscle necrosis, metabolic disorders, bacterial and viral infections, heat-induced (malignant hyperthermia, heat intolerance, heat stroke), inflammatory, certain drugs (overuse or overdose) such as cocaine, amphetamines, statins, heroin, PCP, as well as low phosphate levels.[8]
Below is a chart that describes the risk factors for rhabdomyolysis as well as examples of the risk factors and associated signs and symptoms. </span>
Risk Factors For Postoperative Rhabdomyolysis[edit | edit source]
- Male
- Age > 10 years
- BMI > 55 kg/m2
- History of hypertension, diabetes mellitus, or peripheral vascular disease
- History of statin use
- Elevated preoperative serum CPK level
- Operation duration > 5hours
- Anesthesia time > 6 hours
- Inadequate hydration
- Urine output < 1.5ml/kg/h
- Bleeding and/or hypotension
- Use of propofol and/or succinylcholine
- Complaints of muscle pain and weakness
- Delayed ambulation
- Urine output <1.5mL/kg/h
- Serum CPK > 1,000IU/L
- Urine myoglobin > 250m g/L
Characteristics/Clinical Presentation[edit | edit source]
The signs and symptoms of rhabdomyolyis vary from person to person. The three most common signs and symptoms are muscle pain, weakness, and dark urine.[2][3] Muscle pain as well as weakness and tenderness may be general or specific to muscle groups. The calves and low back are the most general muscle groups that are affected.[2] According to the author Efstratiadis, back pain and limb pain are the most frequent sites in patients with rhabdomyolysis.[3] However, over 50% of the patients with rhabdomyolysis may not complain of muscle pain or weakness.[2] The initial sign of rhabdomyolysis is discolored urine which can range from pink to dark black.[2][3] Other signs and symptoms include, local edema, cramps, hypotension, malaise, fever, tachycardia, nausea and vomiting.[2][3] Often during the early stages of rhabdomyolysis the following conditions may also be present: hyperkalemia, hypocalcemia, elevated liver enzymes, cardiac dysrrhythmias and cardiac arrest.[2] Some late complications include acute renal failure and disseminated intravascular coagulation.[2]
Complications Associated with Rhabdomyolysis[5][edit | edit source]
Acute Renal Failure
Disseminated Intravascular Coagulation
Electrolyte and Metabolic Derangements
Hypoalbumin
Hypocalcemia (early)
Hypercalcemia (late)
Hypernatremia
Hyperphosphatemia
Hyperuricemia
Cardiac Dysrhythmias
Compartment Syndromes
Shock
Death
Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
Blood samples are taken from the patient to look at various serum values, one of the most important serum indicators of myocyte injury is creatinine kinase.[3]
Creatinine Kinase
Under normal conditions, CK levels are 45-260 U/L. After rhabdomyolysis, the levels of CK can be raised to 10.000-200.000 U/L or even 3.000.000.000 U/L. No other condition except rhabdomyolysis can cause such extreme CK elevation.”[3]
Creatine Kinase has several forms that include the muscles, heart, brain and kidneys, as well as mitochndria so it is important to look at all values.
Uric Acid
Uric Acid is important to check due to the fact that rhabdomyolysis breaks down skeletal muscle creating more creatinine, which then becomes creatinine which can then lead to acute renal failure, therefore causing the levels of uric acid to rise.
Urinalysis
Urine analysis can be very helpful in diagnosing rhabdomyolysis. Urinalysis will be able to detect changes in the body’s waste, such as increases in uric acid, albumin, as well as myoglobin.[3] Often patients that are positive for rhabdomyolysis have brown tinted urine. Table 3 has a description of common findings in urinalysis.
Causes of Reddish-Brown Discoloration of the Urine[4]
Myoglobinuria
Rhabdomyolysis
Traumatic
Non-Traumatic
Hemoglobinuria
Hemolysis
Mechanical Damage
Immunologic Damage
Structural Fragility of Erythrocytes
Microangiopathy
Hematuria
Renal Causes
Postrenal Causes
External Factors
Red Beets
Drugs
Vitamin B12
Rifampicin
Phenolphthalein
Phenytoin
Metabolites
Bilirubin
Porphyrin
Systemic Involvement[edit | edit source]
Systemic involvement for rhabdomyolysis includes the muscle groups that have been directly involved such as during a crush injury or overuse. Once the breakdown of muscle occurs the by-product will then filter into the renal system, which if gone untreated can lead to renal failure.
Medical Management (current best evidence)[edit | edit source]
The best medical management for rhabdomyolysis is stabilizing the patient and aggressive fluid replacement with saline to preserve renal function.[2][3] It is also suggested that fluids be given to victims before extraction. The increase in fluids helps to expand the intravascular volume, thereby inducing diuresis and clearance of toxins.[3] It Is recommended that patients should be given 10 or more liters of fluid per day, so that they maintain a urine output of 150-300 ml/per hour.[3] Sometimes mannitol and bicarbonate are given during the initial resuscitation. It is believed that mannitol acts as a free-radical scavenger minimizing cell injury. Mannitol is also a renal vasodilator to prevent renal failure. Bicarbonate is given to help correct the effects of metabolic acidosis and enhance myoglobin.[3] Along with the patient’s vital signs and urine output, the patient’s electrolytes should be closely monitored.
Prehospital Care[5]
- If Rhabdomyolysis is suspected, establish peripheral access and begin IV rehydration with normal saline
Initial Hospital Stabilization[5]
- Supportive care: ABC measures; treat associated life threatening injuries
- Confirm/establish diagnosis with history, physical examination, laboratory studies (eg. creatine kinase, creatinine, electrolytes, etc.)
- Rehydrate aggressively with normal saline at 10-15 mL/kg/hr to achieve urinary output of 2 mL/kg/hr; switch to hypotonic saline after resuscitation is complete
- Continue rehydration for first 24-72 hours in moderate to severe cases or until patient is hemodynamically stable
- In moderate to severe cases with risk of progression to acute renal failure, preexisting renal disease, or evidence of metabolic acidosis and dehydration, consider urinary alkalinization. The goal urine pH of > 6.5 is achieved by adding 3 ampules of sodium bicarbonate to 1 L of 5% dextrose in water; the solution is infused at an initial rate of 100 mL/hr
- In the nonoliguric patient, consider mannitol 1g/kg IV over 30 min, followed by 5 g/hr IV, for a total of 120 g/day; use mannitol to assist diuresis only in patients who have received adequate volume replacement
- Monitor for and treat hyperkalemia aggressively
- Monitor urinary output and renal function closely
- Monitor for coagulopathy, compartment syndrome, and sepsis in severe cases
- Consider hemodialysis in conjunction with a nephrologist for:
Fulminant renal failure with uremic encephalopathy
Uremic pericardial effusion with tamponade physiology
Refractory hyperkalemia, volume overload, or metabolic acidosis
- Attempt to identify the inciting factor and stop further muscle damage and disease progression
Disposition[5]
- Mild to moderate cases with stable electrolytes that are responding to rehydration, admit to a general medicine ward
- In patients with electrolyte abnormalities or underlying cardiac or renal disease, admit to a monitored bed
- In severe cases, including those with fulminant renal failure with sequelae (pulmonary edema, symptomatic hyperkalemia, oliguria/ anuria), persistent hypotension, or DIC, admit to intensive care unit.
Dialysis
Unfortunately patients that have rhabdomyolysis are more likely to develop acute renal failure. A common treatment for acute renal failure is dialysis to correct fluid, electrolytes, and acid-base abnormalities. This is a slow process to correct the fluid overload and as well as removal of potassium and urea.[2]
Medications[edit | edit source]
A patient wil rhabdomyolysis will not take medications on a regular basis, they will only take them in the emergency medical treatment. However, patients are encouraged to drink lots of water throughout treatment.
Physical Therapy Management (current best evidence)[edit | edit source]
It is important to keep in mind the cause of rhabdomyolysis. It is important to not overexert the patient to prevent them from creating more muscle breakdown. The most important thing is for the patient to retain range of motion as well as to properly hydrate.
The physical therapist treating a patient with rhabdomyolysis must make sure that the patient is not having any urinary problems which includes urine color.[9] Some interventions would include range of motion exercises both active and passive, aerobic training, and gradual resistance training.[9]
Differential Diagnosis[edit | edit source]
Most Common Differential Diagnoses[10]
- Burns, Electrical
- Carnitine Deficiency
- Child Abuse and Neglect, physical abuse
- Dermatomyositis
- Multisystem Organ Failure of Sepsis
- Myoglobinuria[4]
- Neuroleptic Malignant Syndrome
- Sepsis
- Systemic Inflammatory Response Syndrome
- Systemic Lupus Erythmatosus
- Thromboembolism
- Toxic Shock Syndrome
- Toxicity, Ethanol
Other Problems to Consider[10]
Traumatic injuries , Viral infections, Myalgias from other etiologies, Bacterial infections, Pyomyositis, Heatstroke , Cold exposure, Snakebite, Malignant hyperthermia, Muscle phosphorylase deficiency, Phosphofructokinase deficiency, Carnitine palmityl transferase deficiency, Phosphoglycerate mutase deficiency, Other inborn errors of metabolism, Hyperosmotic conditions, Guillain-Barré syndrome, Inflammatory myositis.
Case Report (Diagnosis and Treatment of Acute Exertional Rhabdomyolysis)[11][edit | edit source]
Subjective Findings[edit | edit source]
Patient was a walk in to the US Military Academy Cadet Physical Therapy Clinic complaining of bilateral shoulder pain and weakness. The patient reported performing hundreds of push up of varying types 36 hours earlier. The patient reported being in pain and that he had noticed dark colored urine 24 hours after his push up session.
Objective Findings[edit | edit source]
Range of Motion
Bilateral shoulder AROM restricted below 90 degrees elevation with abnormal scapulohumeral rhythm (excessive scapular elevation bilaterally)
PROM was within normal limits but caused pain in the pectoralis and triceps near end range shoulder elevation
Left elbow AROM and PROM were restricted to 90 degrees flexion due to pain and induration in the left triceps.
Strength
Shoulder ER 3+/5
All other muscle strength assessment was deferred due to the severity and irritability of the patient's symptoms
Special Tests
Shoulder impingement tests were negative (Hawkins-Kennedy and Neer)
Exquisite tenderness to palpation bilaterally in the pectoralis, triceps, and infraspinatus muscle, as well as in the bicipital groove
Laboratory Tests
Serum CK, completed blood count, and urinalysis were ordered
Serum CK was listed as 9,600 U/L (normal range 55-170 U/L)
Urinalysis noted that the urine was brown
Management[edit | edit source]
Patient was diagnosed with Acute Exertional Rhabdomyolysis and was admitted to the hospital. He was given aggressive fluid replacement. While in inpatient he performed AAROM in shoulders and in elbows. Patient was discharged after 4 days and then returned to the outpatient clinic.
Outpatient
Randall et al's Rehabilitation program for patients with with acute exertional rhabdomyolysis secondary to intense push-up training
Phase 1
Active and gentle passive ROM of the shoulder and elbow within limits of pain
Phase 2
Initiated once active ROM is normal. Upper body ergometer at low intensity for 5 minutes progressing daily until this workload can be maintained for 15 minutes.
Phase 3
Initiated once the patient can maintain 15 minutes, on the upper body ergometer without discomfort, change in technique, or muscle soreness 24 hours post exercise. Progress to isotonic weight training with light weights for specific muscle weakness (ex. elbow extension for triceps), modified pushups, and bench press. Modified pushups are performed daily on an incline (such as against a wall) and progressed as tolerated to tabletop, stool, and floor (without modification).
Phase 4
Initiated once patient progresses to pushups without modification. Patient is allowed to resume normal exercise routine with the restriction of only performing 1 set of pushups in any 24 hour period. This restriction in maintained until the patient is able to perform at their preinjury number of pushups without sequelae such as muscle soreness or loss of normal ROM.
Outcome[edit | edit source]
First Outpatient Visit
Shoulder AROM:
Flexion 155 degrees
Abduction 170 degrees
External Rotation 55 degrees
Internal Rotation 65 degrees
Strength
5/5 for shoulder shrug, internal rotation, and elbow flexion
4/5 or 4-/5 for shoulder abduction, flexion, external rotation, supraspinatus and triceps
CK level 5,721 U/L
Eight Day Post Diagnosis
Full AROM
Began Radall et al.Protocol
Thirty-Seven Days Post Diagnosis - serum CK levels normal
Seventy- One Days Post Diagnosis - patient's full strength was back and was able to perform 60 pushups in one continuous bout
Resources
[edit | edit source]
add appropriate resources here
Recent Related Research (from Pubmed)[edit | edit source]
Failed to load RSS feed from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/erss.cgi?rss_guid=1faJNdFX7yLhGzofhk71uteNlm0H6NA44FONqsgXwabBiebkzh|charset=UTF-8|short|max=10: Error parsing XML for RSS
References[edit | edit source]
- ↑ 1.0 1.1 Goodman CC, Fuller KS. Pathophysiology: Implications for the Physical Therapist. 3rd ed. St. Louis, MO: Saunders-Elsevier; 2009
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Huerta-Alardin AL, Varon J, Marik P. Bench-to-beside review: Rhabdomyolysis - an overview for clinicians. Critical Care 2005; 9: 158-169
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 Efstratiadis G, Voulgaridou A, Nikiforou D, et al. Rhabdomyolysis updated. Hippokratia 2007; 11(3): 129-137.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Vanholder R, Mehmet S, Erek E, Lameire N. Rhabdomyolysis. Journal of the American Society of Nephrology 2000; 1553-1561.
- ↑ 5.0 5.1 5.2 5.3 5.4 Walter LA, Catenacci MH. Rhabdomyolysis. Hospital Physician 2008; 25-31.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Bagley WH, Yang H, Shah KH. Rhabdomyolysis. Intern Emergency Medicine 2007; 2: 210-218
- ↑ Savage DCL, Forbes M. Idiopathic Rhabdomyolysis. Archieves of Disease in Childhood 1971; 26: 594-607
- ↑ 8.0 8.1 8.2 8.3 8.4 Tanaka PP, Brodsky J. Rhabdomyolysis Following Bariatric Surgery. Bariatric Times 2007.
- ↑ 9.0 9.1 Brown T. Exertional Rhabdomyolysis: Early Recognition is Key. The Physician and Sports Medicine 2004; 32: 1-5
- ↑ 10.0 10.1 Muscal E. Rhabdomyolysis: Differential Diagnoses and Workup. eMedicine 2009.
- ↑ Baxter R, Moore J. Diagnosis and Treatment of Acute Exertional Rhabdomyolysis. Journal of Orthopaedic and Sports Physical Therapy 2003; 33(3): 104-108