Methicillin-Resistant Staphylococcus Aureus
Original Editors - Students from Bellarmine University's Pathophysiology of Complex Patient Problems project.
Top Contributors - Kory Reynolds, Sarah Tassinari, Lucinda hampton, Elaine Lonnemann, 127.0.0.1, Kim Jackson, Evan Thomas, WikiSysop and Nupur Smit Shah
Introduction[edit | edit source]
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium that causes infections in different parts of the body. It's tougher to treat than most strains of staphylococcus aureus -- or staph -- because it's resistant to some commonly used antibiotics.
MRSA infection is one of the leading causes of hospital-acquired infections and is commonly associated with significant morbidity, mortality, length of stay, and cost burden. MRSA infections can be further divided into hospital-associated (HA-MRSA) infections and community-associated (CA-MRSA) infections. They differ not only in respect to their clinical features and molecular biology but also to their antibiotic susceptibility and treatment
The bacteria from which MRSA arises, staphylococcus aureus, is found in the skin and in the nostrils of one third of all people and do not show any symptoms of having been exposed to the bacteria. These carriers of the bacteria are then exposing the bacteria to all of the items that they touch as well as expelling it into the air where it will remain until the item is next cleaned.[1]
Etiology[edit | edit source]
Methicillin resistance has occurred in S. aureus by mutation of a penicillin-binding protein, a chromosome-encoded protein. This type of resistance is transferred between S. aureus organisms by bacteriophages. This is one of the only medically relevant examples of chromosome-mediated drug resistance by phage transduction.
Epidemiology[edit | edit source]
The history of MRSA infection goes back to 1961 when it was first described. Since then, the incidence and prevalence of MRSA infection have been increasing dramatically across the United States. Recently, some population studies have hinted at reducing HA-MRSA incidences in the United States but at the expense of a growing prevalence of CA-MRSA. The reported incidence of MRSA infection ranges from 7% to 60%.
Risk Factors
- The commonly associated risk factors for MRSA infection are prolonged hospitalization, intensive care admission, recent hospitalization, recent antibiotic use, MRSA colonization, invasive procedures, HIV infection, admission to nursing homes, open wounds, hemodialysis, and discharge with long-term central venous access or long-term indwelling urinary catheter.
- A higher incidence of MRSA infection is also seen among healthcare workers who come in direct contact with patients infected with this organism.
- Although advancing age by itself is not considered a risk factor for MRSA infection, age more than 65 years is a significant risk factor for hospitalization.
- Living in an area with a high prevalence of CA-MRSA or admission to a hospital with a high prevalence of HA-MRSA also is considered a significant risk factor for MRSA colonization.
Pathophysiology[edit | edit source]
The key reason for MRSA resistance to beta-lactam antibiotics is due to the presence of the mecA gene sequence, which is known to generate transpeptidase PB2a that lowers the affinity of the organism to bind to beta-lactam antibiotics.[3]
Clinical Presentation[edit | edit source]
MRSA can cause a range of organ-specific infections, the most common being the skin and subcutaneous tissues, followed by invasive infections like osteomyelitis, meningitis, pneumonia, lung abscess, and empyema. Infective endocarditis caused by MRSA is associated with an increased morbidity and mortality compared to any other organism and is linked to intravenous drug abuse.
Skin and soft tissue infections (SSTI): CA-MRSA is a predominant organism associated with SSTIs like cellulitis, necrotizing fasciitis, and diabetic foot ulcers. It also is increasingly associated with more invasive disease than those due to non-MRSA. More frequently these infections are multidrug-resistant leading to frequent recurrence, increased hospitalization, and mortality[5][8].
Bone and joint infection: Staphylococci are the most common cause of bone and joint infections. Oxacillin resistance has become increasingly common among these patients. MRSA can cause osteomyelitis of spine, long bones of upper and lower extremities by extension of local infection from a wound or as a part of hematogenous infection. Similarly, MRSA can cause septic arthritis of both native and prosthetic joints.
Pneumonia: Staphylococcal pneumonia, historically known, as post-influenza pneumonia, was a distinct clinical entity with a dramatic onset of respiratory symptoms and mortality ranging from 80% to 90% in the pre-antibiotic era. It carried specific radiological features including cavitary lesions, empyema, and pyopneumothorax and pathological features such as pulmonary hemorrhage and microabscess formation. In the post-antibiotic period, the course has been less explosive, not always associated with viral influenza, associated with other risk factors for S. aureus infections, and carries a mortality of around 30% to 40%. Nevertheless, CA-MRSA causing life-threatening necrotizing pneumonia in otherwise healthy individuals has been reported across the United States recently. It is characterized by severe respiratory symptoms, high fevers, hemoptysis, and hypotension. It rapidly progresses to sepsis and septic shock with leukopenia and elevated C-reactive protein (greater than 350 mg/dL). Multilobar cavitating alveolar infiltrates in a clinical setting like this is consistent with CA-MRSA infection.
MRSA is also a leading cause of hospital-acquired and ventilator-associated pneumonia. Hospital-acquired pneumonia (HAP), or nosocomial pneumonia, is characterized as pneumonia developing 48 hours or more after hospital admission, indicating that it was not incubating at the time of admission. Ventilator-associated pneumonia (VAP) is defined as pneumonia developing 48 hours or more after implementation of endotracheal intubation and mechanical ventilation and was not present before intubation. The microbiological etiology of these two conditions is similar and carries grave prognosis associated with poor overall outcomes.
Bacteremia: Bacteremia due to S. aureus has been reported to be associated with mortality rates of 15% to 60%. MRSA bacteremia is commonly seen in intensive care unit patients with central line insertions. Infective endocarditis is associated with MRSA bacteremia and should be ruled out in any patient with MRSA in the bloodstream. The outcomes related to MRSA bacteremia are worse than other MRSA infections because of the decreased response to vancomycin in these patients.
Endocarditis: MRSA is an important cause of bacterial endocarditis which can cause mortality in about a third of the infected patients (30-37%). Right-sided MRSA endocarditis is commonly associated with intravenous drug use and intravenous catheters. Patients with tricuspid valve vegetations may have septic pulmonary emboli causing nodular infiltrates and cavitating lesions in the lungs. Similarly, patients with involvement of mitral and aortic valves may have secondary infections in distant foci such as bones and joints, kidneys, brain, and other organs. It is important to take history and perform a thorough examination of these patients combined with necessary labs and radiological tests.
Characteristics/[edit | edit source]
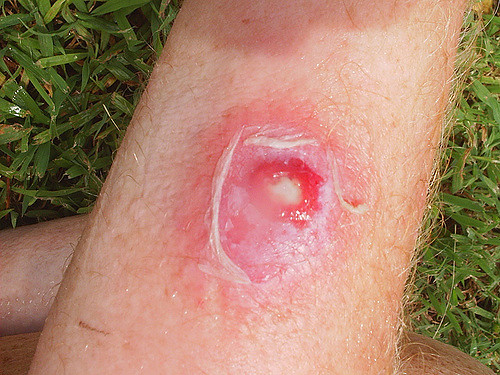
A MRSA infection can start as a swollen, painful red bump that resembles a pimple or a spider bite. Observing the area, the infection may be warm to the touch, full of drainage, and could be accompanied by a fever.[2] These infections can quickly spread into deep, painful abscesses that will require surgical draining. They also have the potential to spread infections to the bones, joints, surgical wounds, bloodstream, heart valves, and lungs, which could be life-threatening.[2] When evaluating these patients, be aware of the following symptoms, for they are indicative of a severe infection: [3]
• Chest pain
• Cough
• Shortness of breath
• Fatigue
• Fever and chills
• General ill feeling
• Headache
• Rash
• Wounds that do not heal
Environmental Risk Factors for Exposure to MRSA include:[4]
• Hospitalization overnight or long-term care facility
• Undergoing invasive procedures
• Work/Live in overcrowded/poor sanitary conditions
Personal Risk Factors for Acquisition of MRSA include: [4]
• Skin disorders
• Wounds
• Transcutaneous/indwelling medical devices
• Indwelling urinary catheter
• Diabetes Mellitus
• Immunodeficiency
• Intravenous drug abuse
• Practice of contact sports
Differences in Characteristics Between HA-MRSA and CA-MRSA[5]
| Characteristic | HA-MRSA | CA-MRSA |
| Site of Infection | Bacteraemia, wound infections, respiratory tract, urinary tract | Mainly skin (abscesses, cellulitis, furunculosis |
| Risk Factors | Indwelling devices, catheters, lines, hemodialysis, prolonged hospitalization, long-term antibiotic use | Close physical contact, abrasion injuries, poor hygiene |
| Transmission | Person-to-person (healthcare staff, visitors, patients), environment-to-person (hospital equipment | Person-to-person (contact sports), environment-to-person (shared facilities, shared sports equipment) |
Associated Co-morbidities[edit | edit source]
Characteristics of Patients at Time of Detection/Identification of MRSA[6][7]
- Heart Disease
- Asthma
- Diabetes Mellitus
- End-Stage Renal Disease
- End-Stage Liver Disease
- Immunocompromised, Non-cancer
- Solid-Organ Cancer
- Hematologic Malignancy
Medications[edit | edit source]
Typically, cases of HA-MRSA do not respond to methicillin or other antibiotic agents. However, majority of cases of CA-MRSA are susceptible to other antibiotics to rid of the bacteria.[5] Lab results will lead the doctor to prescribe a specific antibiotic for each specific case if needed.[3] Teicoplanin and vancomycin are two of the most common antibiotics used in treatment.[8] In some cases, though, antibiotics are not necessary. Simply draining the wound/abscess will be sufficient treatment to prevent the spread of MRSA.[2]
Diagnostic Tests/Lab Tests/Lab Values[edit | edit source]
Clinical Laboratories for MRSA, according to the Clinical and Laboratory Standards Institute (CLSI)[9]
Broth Microdultion Testing with the addition of one of the following:
- A plate containing 6 μg/ml of oxacillin in Mueller-Hinton agar supplemented with 4% NaCl as alternative methods of testing for MRSA
- Latex Agglutination Test for PBP2 - tests for the mecA gene (Staphylococcal resistance to oxacillin/methicillin happens when an altered penicillin-binding protein, PBP2a, is produced.)
- Cefoxitin Disk Screen Test
Typically the broth test and agar test are the main tests for detecting MRSA, and the cefoxitin disk screen test can be used as a back-up test.
Interpretation of Susceptibility of Staphylococci to Oxacillin
| Susceptible | Resistant | |
| Oxacillin MIC Test | S. Aureus ≤ 2 μg/ml CoNS ≤ 0.25 μg/ml |
S. Aureus ≥ 4 μg/ml CoNS ≥0.5 μg/ml |
| Cefoxitin MIC Test | S. Aureus ≤ 4 μg/ml CoNS N/A |
S. Aureus ≥ 8 μg/ml CoNS N/A |
| Cefoxitin Disk Diffusion Test | S. Aureus ≥ 22 mm CoNS ≥ 25 mm |
S. Aureus ≤ 21 mm CoNS ≤ 24 mm |
• Detecting oxacillin/methicillin resistance can be difficult because of the presence of multiple populations (susceptible and resistant) may coexist within one culture of staphylococci.
• Oxacillin and cefoxitin are tested instead of methicillin because methicillin isn’t as readily available in the US as it once was. Oxacillin detects subpopulations well and cefoxitin activates mecA gene better, making it a more accurate test than other using oxacillin.
• Methicillin and oxacillin are in the same class of drugs. Methicillin has historically been the drug of choice for detecting resistance, hence the name MRSA. Even though the preferred drug of choice for testing has changed, the name had remained the same.
According to Centers for Disease Control and Prevention, there are additional tests to detect oxacillin/methicillin resistance but the most common and reliable tests have been listed above.
Etiology/Causes[edit | edit source]
The bacteria Staphylococcus aureus lives on the skin of one third of the population who do not present with any symptoms.[1] However, they still have the ability to spread the bacteria through touch and through the air, contaminating their surroundings without the knowledge of doing so. Most staph infections are then spread by skin-to-skin or equipment-to-skin contact.[3] Healthcare workers' hands are at high risk for cross-infection in patients who develop or are exposed to HA-MRSA, making hand hygiene highly important in minimizing the risk of contracting the bacteria.[10] Those who are at risk of acquiring HA-MRSA and CA-MRSA are listed above in the Characteristics/Clinical Presentation section.
Systemic Involvement[edit | edit source]
MRSA Infection may affect but are not limited to:[3]
- Bloodstream
- Lungs
- Heart
- Bones
- Joints
Please refer to the ‘Characteristics/Clinical Presentation’ section for more information on the associated signs and symptoms.
Medical Management[edit | edit source]
A common practice to rid the body of MRSA infection is known as decolonization. The purpose of this treatment is to remove MRSA from the patients’ skin and nostrils with an intranasal ointment as well as an antimicrobial body wash.[1] The patient must use these for five consecutive days to decrease further transmission and infection. However, 21% of patients undergoing this treatment will fail due to non-adherence.[1] These patients are mainly under the care of a primary care physician in an outpatient clinic. Treatment is more likely to be successful in the hospital setting due to the nurses and cleaning staff managing the environment. If the treatment continues to fail after two attempts, the patient is deemed as “chronically colonized”. These patients will be treated only for risk assessment (i.e. during surgery) to prevent re-colonization.[1]These patients must change their lifestyles by regularly cleaning their home, bedding, towels, and clothes.
Some strands of MRSA (identified through blood work) have the ability to be treated through antibiotics. Teicoplanin, Vancomycin, and other antibiotics are often prescribed when the physician feels the infection will benefit from pharmacological intervention.[8]
Presently, the research is looking towards preventative strategies to decrease the incidence of MRSA in the healthcare settings. Nurses are the healthcare workers in most contact with the patients in the hospitals and therefore, can be cross-contaminating patients with MRSA bacteria if there hand hygiene is deficient. In some areas, hand-washing rate in nurses has found to be as low as 13-20% from patient to patient.[10] Chun et al. demonstrates that proper hand hygiene during nursing care can directly reduce the number of infections in the hospital setting. For example, a hospital’s MRSA acquisition rate decreased from 13.3 per 1,000 patients to 2.0 per 1,000 patients simply by changing their hand-washing criteria for the nursing staff.[10] Hand hygiene is the easiest, cost-effective way to decrease the infection rate of our patients in the healthcare settings.
Physical Therapy Management[edit | edit source]
MRSA is not primarily managed by physical therapy. Instead it is the physical therapist’s role to identify comorbidities that are inhibiting functional activity and treat the patient’s symptoms. When treating a patient with MRSA, it’s important for the physical therapist to know proper disinfecting techniques to prevent the spread of the disease. Precautions to be taken when treating a patient with MRSA include but are not limited to:[11][12][3]
- Buy disinfectants that are registered by the Environmental Protection Agency (EPA)
- Note how to properly apply each product, how long it needs to be left on the surface, if the surface needs to be rinsed prior, if it’s only for specific surfaces, etc.
- Laundering specific clothes, towels and linens separately would be appropriate but not always necessary – wash and dry in the warmest temperate recommended on each piece of linen individually
- All surfaces ad equipment that came in contact with the patient should be cleaned, sometimes individual equipment for the patient with MRSA may be necessary if equipment cannot be properly cleaned
- Use barriers between the patient’s skin and surface when possible
- Physical therapist should wash body parts immediately after making contact with patient’s skin
- Patient should keep all open wounds clean and covered at all times until healed
There has also been evidence to support the use of low-frequency ultrasound on bacteria, including MRSA. According to an article posted in 2010, low-frequency ultrasound delivered at 35 kHz reduced colony forming units of bacteria and alters colonial characteristics of MRSA.[13]
Differential Diagnosis[edit | edit source]
The differential diagnosis for MRSA includes the following infections:[14]
• Bacteremia
• Chemical burns
• Impetigo
• Juvenile Idiopathic Arthritis
• Streptococcal Infections
• Kawasaki Disease
• Leptospirosis
• Parvovirus B19 Infection
Further screening should be done to rule out such diseases.
Case Reports/ Case Studies[edit | edit source]
Hörner A, Hörner R, Salla A, Nunes M, Garzon L, Rampelotto R et al. Staphylococcal scalded skin syndrome in a premature newborn caused by methicillin-resistant Staphylococcus aureus: case report. Sao Paulo Med J. 2015;133(5):450-453.
Braich P, Aggarwal S, Mukhtar, BA S, Almeida D. Nonsocomial Keratitis Caused by Methicillin-Resistant Staphylococcus Aureus : Case Report and Preventative Measures. Journal of Community Hospital Internal Medicine Perspectives. 2015;5(5).
References[edit | edit source]
- ↑ 1.0 1.1 1.2 1.3 1.4 Robinson J, Edgley A, Morrell J. MRSA care in the community: why patient education matters. British Journal of Community Nursing. 2014;19(9):436-441. http://dx.doi.org.libproxy.bellarmine.edu/10.12968/bjcn.2014.19.9.436 (accessed 2 April 2016).
- ↑ 2.0 2.1 2.2 Mayo Clinic Staff. MRSA infection - Mayo Clinic [Internet]. Mayoclinic.org. 2016.http://www.mayoclinic.org/diseases-conditions/mrsa/basics/definition/con-20024479 (accessed 1 April 2016).
- ↑ 3.0 3.1 3.2 3.3 3.4 MedlinePlus Medical Encyclopedia. MRSA. https://www.nlm.nih.gov/medlineplus/ency/article/007261.htm (accessed 7 April 2016).
- ↑ 4.0 4.1 Skov R, Gudlaugsson O, Hardardottir H, Harthug S, Jakobsen T, Jørn Kolmos H et al. Proposal for common Nordic epidemiological terms and definitions for methicillin-resistant Staphylococcus aureus (MRSA). Scandinavian Journal of Infectious Diseases. 2008;40(6-7):495-502. http://eds.b.ebscohost.com.libproxy.bellarmine.edu/ehost/detail/detail?vid=12&sid=71ee6b7e-199d-4adc-8e31-bc94eca59a09%40sessionmgr113&hid=112&bdata=JmxvZ2luLmFzcCZzaXRlPWVob3N0LWxpdmUmc2NvcGU9c2l0ZQ%3d%3d#AN=105649710&db=cin20 (accessed 2 April 2016).
- ↑ 5.0 5.1 Millar B, Loughrey A, Elborn J, Moore J. Proposed definitions of community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA). Journal of Hospital Infection. 2007;67(2):109-113. http://eds.b.ebscohost.com.libproxy.bellarmine.edu/ehost/detail/detail?vid=14&sid=71ee6b7e-199d-4adc-8e31-bc94eca59a09%40sessionmgr113&hid=112&bdata=JmxvZ2luLmFzcCZzaXRlPWVob3N0LWxpdmUmc2NvcGU9c2l0ZQ%3d%3d#AN=105828672&db=cin20 (accessed 2 April 2016).
- ↑ Huang S, Hinrichsen V, Datta R, Spurchise L, Miroshnik I, Nelson K et al. Methicillin-Resistant Staphylococcus aureus Infection and Hospitalization in High-Risk Patients in the Year following Detection. PLoS ONE. 2011;6(9):e24340. http://journals.plos.org/plosone/article/asset?id=10.1371%2Fjournal.pone.0024340.PDF (accessed 8 April 2016).
- ↑ Datta R, Huang S. Risk of Infection and Death due to Methicillin‐Resistant Staphylococcus aureus in Long‐Term Carriers. Clinical Infectious Diseases. 2008;47(2):176-181. http://cid.oxfordjournals.org/content/47/2/176.full.pdf+html (accessed 8 April 2016).
- ↑ 8.0 8.1 Choi H, Park K, Park S, Jun B, Lee D, Yeo S. The appropriate medical management of methicillin-resistant Staphylococcus aureus in chronic suppurative otitis media. Acta Oto-Laryngologica. 2010;130(1):42-46. http://dx.doi.org.libproxy.bellarmine.edu/10.3109/00016480902870522 (accessed 2 April 2016).
- ↑ Centers for Disease Control and Prevention. Laboratory Testing for MRSA. http://www.cdc.gov/mrsa/lab/ (accessed 8 April 2016).
- ↑ 10.0 10.1 10.2 Chun H, Kim K, Park H. Effects of hand hygiene education and individual feedback on hand hygiene behaviour, MRSA acquisition rate and MRSA colonization pressure among intensive care unit nurses. International Journal of Nursing Practice. 2014;21(6):709-715.
- ↑ Centers for Disease Control and Prevention. Cleaning & Disinfecting Athlete Facilities. http://www.cdc.gov/mrsa/community/environment/athletic-facilities.html (accessed 8 April 2016).
- ↑ Centers for Disease Control and Prevention. Laundry. http://www.cdc.gov/mrsa/community/environment/laundry.html (accessed 8 April 2016).
- ↑ Conner-Kerr T, Alston G, Stovall A, Vernon T, Winter D, Meixner J et al. The Effects of Low-frequency Ultrasound (35kHz) on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Ostomy Wound Manage. 2010;56(5):32–42. http://www.o-wm.com/content/effects-low-frequency-ultrasound-35-khz-methicillin-resistant-staphylococcus-aureus-mrsa-vit (accessed 9 April 2016).
- ↑ Staphylococcus Aureus Infection Differential Diagnoses [Internet]. Emedicine.medscape.com. 2016. http://emedicine.medscape.com/article/971358-differential (accessed 6 April 2016).