Kienbock's Disease
Original Editors - Fien Selderslaghs, Mirabella Smolders, Liese Magnus, Laura Van Der Perren and Jessica Worrell
Top Contributors - Jessica Worrell, Lucinda hampton, Admin, Laura Ritchie, Naomi O'Reilly, Shreya Pavaskar, WikiSysop, Fasuba Ayobami and Evan Thomas
Definition/Description[edit | edit source]
Kienböck’s disease, also known as lunatomalacia, is an idiopathic avascular necrosis of the lunate bone. In other words, it presumably develops as a reaction of losing blood supply. Two primary theories on the cause of lunate bone ischemia have been submitted: trauma-related or atraumatic.[1]
It is considered an infrequent illness that can possibly develop into carpal collapse if untreated. Robert Kienböck initially characterized this disorder in 1910 as an “Ischemia and necrosis of the lunate bone that occurs in the absence of an acute fracture or non-union.” The cause of Kienböck still remains relatively little known.[2] Kienböck hypothesized that the disorder may be provoked by a disturbed nutrition of the lunate. Following his first description of Kienböck’s disease, diverse research studies have been published in which different correlations were discussed. Lunatomalacia was associated with negative ulnar variance by Hulten, disrupted arterial supply of the lunate by Lee and Gelberman et al and obstruction of venous outflow by Schiltenwolf et al.[3]
Clinically Relevant Anatomy[edit | edit source]
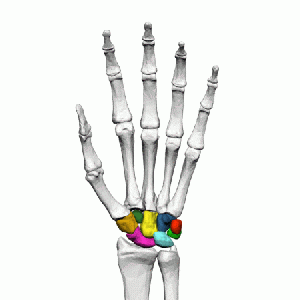
There are eight carpal bones arranged into two rows of four. The carpus is convex posteriorly and concave anteriorly. The lunate is one of four carpal bones in the proximal row, resting between the scaphoid and triquetrum.
Kienböck’s disease corrodes the lunate bone. The lunate has four surfaces which are covered with articular cartilage and have no sites for ligament attachment and have no vascular foramina. Proximally the lunate articulates with the triangular fibrocartilage (TFC) and the distal radius, which transmits 80% of the axial loading through the wrist. Distally, the lunate will generally only articulate with the capitate but in 45% of cases, there will be a second joint surface present (i.e. an articulation with the hamate).[4] Medially, the lunate articulates with the triquetrum and laterally with the scaphoid.[5][6]
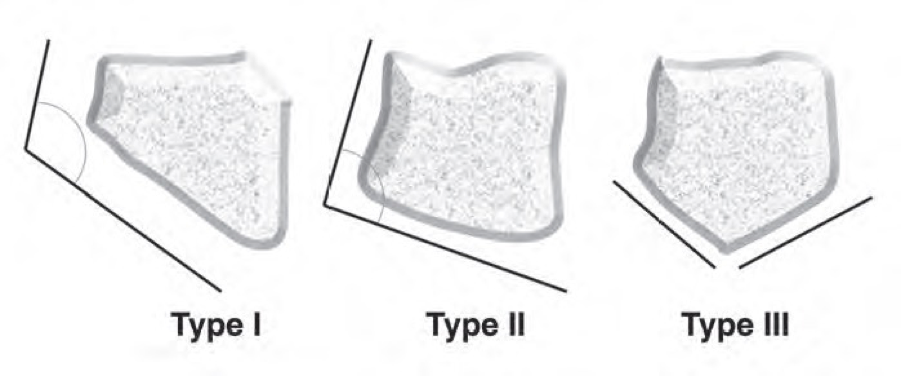
There are three morphologic types of the lunate based on the angle between the lateral scaphoid and proximal radial sides of the lunate, according to the Antuña Zapico classification.[4]
• Type I: the angle is more than 130°
• Type II: the angle is less than 130° (around the 100°)
• Type III: here are two distinct facets on the proximal surface. One articulates with the radius, the other with the triangular fibrocartilage
For blood supply, the lunate bone has extra- and intraosseous components. The extraosseous supply is comprised of vessels that enter the lunate through the palmar and dorsal poles of the bone. The palmar vessels have more foraminal vessels and have thus a greater proportion of blood supply compared with the dorsal vessels.[7]
The dorsal lunate vascularity consists of the dorsal radiocarpal and intercarpal arches. These arches arise from the plexus of vessels, which are located directly over the dorsal pole of the lunate. The palmar lunate vascularity consists of the intercarpal and radiocarpal arches. Through various ligaments, like the radioscapholunate, radiolunate triquetral ligament and ulnar lunate triquetral ligament, the vessels can enter the palmar pole. Between the dorsal and palmar vessels are different patterns of infraosseous anastomosis, which takes care for the intraosseous blood supply. These vessels enter the lunate through the bone foramina.[7]
Epidemiology /Etiology[edit | edit source]
Kienböck’s disease is most common in young adults between 20 and 45 years but can occur in younger and older populations as well. It is more common in men.[8]
The precise etiology of Kienböck’s disease remains uncertain but it has been potentially referred to several factors such as mechanical, traumatic, anatomic and systematic causes.[9] An association can be noted between Kienböck’s disease and an ulnar variance. This change in ulnar variance can be attributed to age, sex and position of the wrist as well as osteoarthritis secondary to Kienböck’s disease. Also morphological factors can play a role in the etiology.[8]
Many theories have been postulated to explain the actual cause resulting in avascular necrosis. It is probably a combination of local vascular and anatomical variations that causes the lunate to develop avascular necrosis.[10] Other factors that can cause avascular necrosis are:
- Single or repetitive microfractures.[11]
- Recurrent compression of the lunate between capitate and distal radius (this disrupts interosseous blood supply).
- Extreme wrist positions or repetitive compression loading of the wrist.[7][12]
- Poor vascularization of the lunate[11]
- Bone geometry of the lunate (Type I) and radius (decreased inclination)[11]
- Negative ulnar variance[11]
It is thought that an increased load through the lunate helps to accelerate the process of fracture and ultimately collapse.[11]
Characteristics/Clinical Presentation[edit | edit source]
Typical symptoms of Kienböck’s disease are activity-related dorsal wrist pain and weakness, often accompanied by swelling dorsally around the lunate. The complaints are in most cases longstanding and increase progressively. The pain can be described from just mild and occasional to severe and debilitating. The range of motion of the joint is nearly always decreased, with loss of flexion and extension. Compared to the unaffected side there is loss of grip strength.[8] Symptoms are most commonly in the dominant wrist and a history of trauma may be present.[11]
The length of the two bones of the forearm, in particular the ulna and the radius, is not usually equally. We can make a subdivision among the length between the distal articular surfaces of the radius and ulna, called the ulnar variance. It may be negative, positive or neutral. Negative ulnar variance indicates that the distal articular surface of the ulna is located more proximally than the radius, positive ulnar variance indicates the stage where the distal articular surface of the ulna is located more distally compared with the surface of the radius and at last neutral ulnar variance means that the ulnar and radial surfaces are at the same level.[13]
According to Lichtmann et al, the Kienböck’s disease can be subdivided into four stages:[4]
| Stage I | May be perceived by radiographic and CT findings that the density and the contour of the lunate bone is quite normal, but according to the MRI findings there can be noticed that some edema can appear in the bone narrow.[4] |
| Stage II | Characterized by compression of the lunate bone, without some significant modification of its contour.[6] This is associated with increased bone density and debilitation of the lunate on radiographic and CT scans.[4] |
| Stage III | Is the most common stage and is identified as a disruption of the lunate,[6] without radiocarpal or midcarpal degenerative arthritis.[4] The third stage can be subdivided in three subcategories:
Stage IIIA and IIIB are difficult to distinguish. It can be categorized by the radioscaphoid angle. If the angle is less than 60°, it can be classified as stage IIIA. If it exceeds 60°, it’s classified as stage IIIB.[6] |
| Stage IV | Stage IV is comparable with stage III. It is characterized by disruption of the lunate and also associated with radiocarpal or midcarpal degenerative arthritis.[4] |
Differential Diagnosis[edit | edit source]
Radiologic imaging, such as MRI and CT-scans, in case of Kienböck’s disease is generally the only way to give a correct diagnosis.[14] Nonetheless, the radiological findings within the lunate bone of Kienböck’s are often very similar to other pathological conditions, which leads to a common misinterpretation.[4] All of the following pseudo lesions are compared to the different stages of this disorder:
1. Lunate Bone Contusion/Acute Fracture
This type of lesion can hardly be differentiated from stage I Kienböck’s Disease. Having a history of acute episodes of severe trauma to the hand is probably the only characteristic of acute lunate bone fracture that can be separated from Kienböck’s.[4]
2. Ulnocarpal Impaction Syndrome
Chronic impaction between the ulnar head, the triangular fibrocartilage complex (TFCC) and the ulnar carpus results in a degenerative pathological condition. Kienböck’s can be differentiated from the Ulnocarpal Impaction Syndrome when there is a bone edema distribution in the ulnar side of the lunate bone, as well as a positive ulnar variance alongside degenerative lesions in the triangular fibrocartilage complex.[4]
3. Infantile and Juvenile Lunatomalacia
This type of Lunatomalacia is acknowledged as the children version of Kienböck’s disease. The only differentiation between these types depends on the age of the patient. Up to 12 years old is called infantile, whereas juvenile affects 13 years and older until skeletal maturity. Pass that point it is called Kienböck’s disease.[4]
4. Arthritis
All sorts of arthritis (rheumatoid, gout, degenerative and post-traumatic) can be compared to the early stages of Kienböck’s. It damages the lunate bone marrow intensity, which can lead to misdiagnosis. However, arthritis has on the one hand both a different clinical presentation and demographic characteristics and default negative ulnar variance on the other.[4][15]
Diagnostic Procedures[edit | edit source]
Diagnostic procedures form the basis for staging, treatment and evaluation of the outcomes.[6] Kienböck’s disease can be diagnosed by evaluating the wrist, along with analyzing the symptoms or any recent injury near the area. Swelling, pain and reduced wrist range of motion are frequent indicators.[14] However, hyperattenuation, density and the degree of the lunate bone collapse are the most important criteria for imaging.[4] A specialist often takes an X-ray of the wrist, in order to gather detailed information and to prevent misdiagnosis. There are many methods to determine Kienböck’s:[4]
- Radiography: Is extremely sensitive and also the most common imaging technique for diagnosing the disease. Plain radiograph can eliminate other pseudo-Kienböck lesions such as fractures and arthritis[4]
- Tomograms: used to determine the extent of the disease
- Bone Scans: help to exclude the presence of Kienböck disease but it is not specific enough to exclude the many other causes of increased uptake in the area of the lunate[11]
- CT-scan: Is claimed to be better and a more specific test than radiographs. This technique is practiced on the late stages of the disease
- MRI: Is declared to be the best imaging technique for Kienböck’s. It is extremely sensitive and specific to detect osteonecrosis.[16] MRI is most significant in the early stages of the disease (particularly in stage I, when the plain radiographs show a rather normal outcome).[4][6]
According to a source, none of these methods were compared to each other. There was additionally no determination to which technique was more accurate. A frequently used staging system for determining radiographic imaging is called “The Stahl classification of Kienbock disease”, which will be discussed below.[4]
Outcome Measures[edit | edit source]
- Range of motion[17][18][19]
- Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire[20][17][18]
- Grip Strength[17][18][19]
- Modified Mayo Wrist (MMW) questionnaire[17][18]
- Pain Scales (VAS/NPR)[17][18][19]
Examination[edit | edit source]
Typically some swelling is visible on the dorsal side of the wrist, along with synovitis.[21] The patient can experience a certain pain situated to the crucifixion fossa, which can be seen between the tendons of both the M. Extensor Digitorum and M. Extensor Carpi Radialis.[5] Furthermore, stiffness and tenderness may develop over the lunate bone.[21]
On physical inspection, the range of motion is quite sensitive, especially when extending the wrist. Movements such as flexion and extension are shrunken, sometimes combined with a progressively loss of grip strength.[5][21] However, the rotation of the forearm is still maintained.
Medical Management[edit | edit source]
The main goal of any therapy for patients with Kienböck’s disease is to improve in wrist pain, range of motion and grip strength. The treatment depends on the level of symptoms therefore the stage of the disease must first be determined.[8]
The treatment of the Kienböck’s disease varies between conservative and surgical interventions. Non-surgical treatment will be implemented in an early stage (stage I) which consists the immobilization of the wrist for three weeks and taking nonsteroidal anti-inflammatory drugs (NSAID’s).[4][22] If the symptoms deteriorate or do not improve after conservative treatment, surgery will be required. Conservative treatment can also be obtained in the second stage when partial necrosis is determined. This includes immobilization for three months. If the patient's presentation does not improve with immobilization, surgery is recommended.[4]
Surgery is particularly suitable because it leads to better outcomes and quicker improvement of the symptoms. It’s considered to improve the range of motion and grip strength by shortening, lengthening or fusing the various bones in the wrist.[4] There are various medical interventions for patients diagnosed with Kienböck’s disease:
| Revascularization | The main goal of revascularization is to improve the blood supply to the lunate. This can be accomplished by bringing a new source of blood supply to the lunate. It is often attempted by vascularized bone graft (also called a pedicle) implanted into the lunate from a nearby bone, usually taken from the radius, pisiform or the lower fibres of the pronator quadratus muscle at the radial styloid process.[4] [23] |
| Capital-shortening osteotomy | Capital-shortening osteotomy includes shortening of the metacarpal bone.[4] |
| Joint levelling |
This is one of the most common techniques used, it reduces the load on the lunate. [24] It can be subdivided in two categories:
An incision is made on the palmar side of the wrist at the height of the ulna. Pins are being placed on both side of the corticotomy, if the lengthening was up to 2 mm. If the lengthening exceeds 2 mm, additional pins will be placed to prevent rotation. [25]
Two parallel transverse incisions are made to eliminate a segment of the radius in order to obtain the same level of the surfaces of the ulna and the radius. A five- or six-hole dynamic compression plate takes care of fixation of the bone. [26] Several studies suggest that the outcomes of radial shortening are better than the ulnar lengthening.[6] |
| Intercarpal fusion | The different purposes of intercarpal fusion are to retain carpal height, to sustain the scaphoid in its proper position and to unload the lunate.[6] There are three types of intercarpal fusion; Capitate-Hamate, Scapho-Trapezial-Trapeziodal and Scapho-Capitate fusion. Capitate-Hamate fusion is the most frequently used technique.[24] |
| Lunate Excision | Lunate excision with or without replacement such as lunate excision, excision with soft-tissue replacement, or silicone replacement arthroplasty[23] |
| Carpectomy | The surgeons will make a dorsal longitudinal or transverse incision through the third dorsal compartment. The lunate is excised first because it is the most accessible. Triquetrum and scaphoid are then excised if possible; if not, both will be gradually removed. [27] A proximal row carpectomy is considered a salvage procedure.[23] |
| Wrist denervation | The intention of this operation is to decrease the pain without loss of hand function and to conserve the mobility of the wrist. There are different ways to perform the operation, varying the incisions and extent of denervation. One of the options is a dorso-radial incision proximal to the distal radio-ulnar joint. Approximately three centimeters of the posterior interosseous nerve (located adjoining to the artery) is excised. [28] |
The various stages are treated by another surgical intervention:
| Stage | Treatment |
| I | Revascularization or capital-shortening osteotomy will be executed in both types of ulnar variance if the conservative treatment isn’t effective.[4] |
| II | When negative ulnar variance is determined, Schuind et al suggest that the surgical treatment for this stage includes joint levelling.[6] This will immediately be implemented when there is complete necrosis established.[4]
Contrary to the negative variance, revascularization can be a cure for patients with positive or neutral variance, when there is complete necrosis of the lunate. It can also be treated by capital-shortening osteotomy.[6] |
| IIIA |
The best option to cure the Kienböck’s disease at this stage, includes joint leveling. This solution will be performed with negative ulnar variance.[4] A patient with positive or neutral variance will be treated by capitate shortening or revascularization at this stage.[4] |
| IIIB | Formerly intercarpal fusion was used to treat the third stage of Kienböck’s disease, but because of the loss of movement that this treatment entails, capitate lengthening after excision of the lunate is recommended.[6] |
| IIIC | Both negative and positive ulnar variance can be resolved by lunate excision and arthrodesis, or proximal row carpectomy.[4] |
| IV | The suitable solutions for the last stage are proximal row carpectomy, denervation of the wrist, arthrodesis or total wrist fusion.[4] Occasionally this last stage can be associated with the carpal tunnel syndrome, which can be handled by decompression.[6] |
Physical Therapy Management[edit | edit source]
Physical therapy is not a common treatment for this disease. A few studies investigated the efficacy of a physical therapeutic intervention following surgery. One study combined a bone marrow transfusion (medical), low-intensity pulsed ultrasound (PT) and an external fixation (surgery). For most of the patients in this study, wrist pain improved to a pain-free level, wrist flexion improved and average grip strength increased. This method can be used as a less invasive surgical alternative for Kienböck’s disease compared to the surgical procedures listed above.[29] Subsequent research is required to confirm the treatment effects found by these authors.
Another study compared different therapies after surgery. One group was treated according to a treatment protocol consisting of kinesiotherapy, electrotherapy, thermotherapy, massage therapy, therapeutic activities and domiciliary guiding. The other group was treated by non-specialized Departments in Hand Therapy and received only thermotherapy and exercises without a direct guidance from the therapist. After therapy, 90% of group one were satisfied with the results of the treatment and 66% of the second group. Other variables such as pain, muscular strength, prehension force, forearm articular range of motion for pronation and supination movements, wrist articular range of motion in abduction and adduction movements and manual function performance were also evaluated. Group one scored better than the second group but again, further research is needed to confirm these findings.[30]
A third study is a case study, reported on a 23 year old professional badminton player who had limited and painful wrist movements and was subsequently diagnosed with Kienböck’s disease. In this case study, the patient was treated surgically. For five weeks after the surgery, the wrist was splinted at all times and local anti-inflammatory and ice were used. Over the next eight weeks, stretching was increased gradually. Regular massage to the wrist extensor and flexor muscles was also done to decrease tone, which can affect joint function. At four months post-surgery, the wrist had painless 0-45° range of extension and 0-70° range of flexion. The patient started his regular training program with tape on his wrist for support. This is a case study thus the findings cannot be extrapolated to all patient populations.[31]
It is clear that there are too few studies about physical management of this condition.
Key Research[edit | edit source]
Resources[edit | edit source]
Clinical Bottom Line[edit | edit source]
References[edit | edit source]
- ↑ D. Kulhawik et al. Avascular Necrosis of the Lunate bone (Kienböck’s disease) secondary to scapholunate ligament tear as a consequence of trauma – a case study. Polish Journal of Radiology, 2014, 79: 24-26.
- ↑ Morón M et al., Proximal Row Carpectomy for Coexisting Kienböck’s Disease and Giant Intraosseous Ganglion of the Scaphoid: A Case Report and Review of the Literature, Case Reports in Orthopedics, 2014, 1-6.
- ↑ Gupta R et al., Outcome of Kienböck’s disease in twelve cases: a mid-term follow-up study, Singapore Medical Journal, 2014, 55(11): 583-586.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 4.22 4.23 4.24 4.25 4.26 4.27 Arnaiz J et al., Imaging of Kienböck Disease, American Journal of Roentgenology, 2014, 203: 131-139.
- ↑ 5.0 5.1 5.2 Fontaine C et al., Kienböck's disease: la maladie de Kienböck, Chirurgie de la Main, 2015, 34(1): 4–17.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 Schuind F et al., Kienböck’s disease, The Journal of Bone and Joint Surgery, 2008, 90-B: 133-139.
- ↑ 7.0 7.1 7.2 Lamas C et al., The anatomy and vascularity of the lunate: considerations applied to Kienböck’s disease, Chir Main, 2007, 26: 13–20.
- ↑ 8.0 8.1 8.2 8.3 8.4 De Smet L et al., Treatment options in Kienböck’s disease, Acta Orthop. Belg., 2009, 75: 715-726.
- ↑ Innes L et al., Systematic Review of the Treatment of Kienböck’s Disease in Its Early and Late Stages, The Journal of Hand Surgery, 2010, 35(5): 713-717.
- ↑ Martin GR et al., Long-term outcomes for Kienböck’s disease, NCBI, 2013, 8(1): 23-26.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Watson HK, Guidera PM. Aetiology of Kienbock's disease. J Hand Surg [Br]. Feb 1997;22(1):5-7.
- ↑ Yazaki N et al., Bilateral Kienbock's disease. J Hand Surg, 2005, 30(2):133-136.
- ↑ Cerezal L et al., Imaging Findings in Ulnar-sided Wrist impaction Syndromes, RadioGraphics, 2002, 22(1): 105-121
- ↑ 14.0 14.1 Allan CH et al., Kienböck’s Disease: Diagnosis and Treatment, Kienböck’s Disease, Journal of the American Academy of Orthopaedic Surgeons, 2001, 9(2): 128-136.
- ↑ Patel N et al., Osteoarthritis of the Wrist, Osteoarthritis - Diagnosis, Treatment and Surgery, 2012, 171-202
- ↑ Watanabe K, Nakamura R, Imaeda T. Arthroscopic assessment of Kienbock's disease. Arthroscopy. Jun 1995;11(3):257-62.
- ↑ 17.0 17.1 17.2 17.3 17.4 Stahl et al. Journal of Orthopaedic Surgery and Research (2015) 10:133 DOI 10.1186/s13018-015-0276-7
- ↑ 18.0 18.1 18.2 18.3 18.4 Ebrahimzadeh H. M, Moradi A, Vahedi E, Kachooei R A. Mid-term clinical outcome of radial shortening for kienbock disease. J Res Med Sci. 2015 February; 20(2): 146–149
- ↑ 19.0 19.1 19.2 Lindsay Innes, BS, Robert J. Strauch. Systematic Review of the Treatment of Kienböck’s Disease in Its Early and Late Stages. J Hand Surg 2010; 35A:713
- ↑ Martin, G. R., & Squire, D. (2013). Long-term outcomes for Kienböck’s disease. Hand (New York, N.Y.), 8(1), 23–26. http://doi.org/10.1007/s11552-012-9470-9
- ↑ 21.0 21.1 21.2 Lutsky K et al., Kienböck Disease, American Society for Surgery of the Hand, 2012, 37(A): 1942-1952.
- ↑ Meena DS et al., Distraction histiogenesis for treatment of Kienböck’s disease: A 2- to 8-year follow-up, Indian Journal of Orthopaedics, 2009, 43(2): 189-193.
- ↑ 23.0 23.1 23.2 Watanabe T, Takahara M, Tsuchida H, Yamahara S, Kikuchi N, Ogino T. Long-term follow-up of radial shortening osteotomy for Kienbock disease.J Bone Joint Surg Am. 2008 Aug;90(8):1705-11.
- ↑ 24.0 24.1 Inoue G. Capitate-hamate fusion for Kienböck’s disease, Acto Orthop. Scand., 1992, 63(5): 560-562 .
- ↑ Kawoosa AA et al., Distraction osteogenis for ulnar lengthening in Kienböck’s disease, International Ortopaedics, 2007, 31(3): 339-344.
- ↑ Iwasaki N et al., Radial osteotomy for late-stage Kienböck’s disease, The Journal of Bone and Joint Surgery, 2002, 84-B: 673-677.
- ↑ John D et al., Proximal row carpectomy and intercarpal arthrodesis for the management of wrist arthritis, Journal of the American Academy of Orthopaedic Surgeons, 2003, 11: 277-281.
- ↑ Röstlund T et al., Denervation of the wrist joint – an alternative in conditions of chronic pain, Acta Orthop. Scand., 1980, 51: 609-616.
- ↑ Ogawa T et al., A new treatment strategy for Kienböck’s disease: combination of bone marrow transfusion, low-intensity pulsed ultrasound therapy, and external fixation, J Orthop Sci., 2013, 18(2): 230-237.
- ↑ Lima SMPF et al., Rehabilitation of patients with Kienböck disease underwent proximal row carpectomy, Acta Ortop. Bras., 2000;8(2): 83-89
- ↑ Karahan AY et al., Kienbock’s Disease That Manifested in a Badminton Player: Case Report, International Journal of Sports Science 2013, 3(4): 132-134.