Guillain-Barré Case Study: David
Abstract[edit | edit source]
Introduction[edit | edit source]
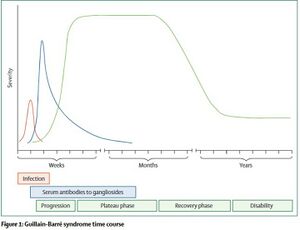
Guillain-Barré Syndrome (GBS) is a rare autoimmune disease that affects the peripheral nervous system (PNS). The exact mechanism is unclear, but the majority of GBS cases are triggered following bacterial or viral infection[1]. GBS can also be triggered by trauma, surgery, cancer, or vaccination[2][3]. Following the triggering event, an autoimmune reaction takes place where the immune system targets and breaks down the myelin sheath surrounding peripheral nerves[4]. The first symptoms noticed are typically numbness, tingling, or pain (alone or in combination) beginning in the hands or feet. The over the course of days to weeks there is progressive muscle weakness in the extremities and potential paralysis[5]. Guillain-Barré Syndrome can also lead to weakening of the respiratory muscles and eventual respiratory failure[1]. Many complications can arise during the acute stage of GBS including: blood clots, heart attack, pneumonia, and infection. Death occurs in about 2.6% of all cases of GBS[6].
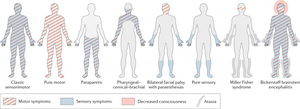
Signs and Symptoms[7][8][9][edit | edit source]
- Numbness, tingling, or pain (alone or in combination) that begins in the hands and feet
- Progressive bilateral weakness of the extremities
- Impaired gait and balance
- Weakness of facial muscles
- Difficulty with swallowing or speaking
- Double vision
- Severe pain that may worsen during the night
- Changes to bowel/bladder control
- Paralysis
- Respiratory failure
- Autonomic dysfunction (abnormal changes to heart rate and blood pressure)
Epidemiology[edit | edit source]
- More common in men[10]
- Risk increases with age[10]
- 3,000 to 6,000 cases per year in the United States[11]
- Incidence of subtypes varies between countries[2]
Subtypes[edit | edit source]
There are several subtypes of GBS, each presenting differently and affecting different populations. In North America, the most common form of GBS is Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP)[2]. AIDP is characterized by the presence of sensory symptoms, muscle weakness, involvement of cranial nerves, and autonomic dysregulation. The focus of this case study is on the AIDP subtype; the presentation follows the classic sensorimotor pattern of symptoms.
Prognosis[edit | edit source]
Symptom severity can vary and as such, the degree of recovery and the timeline also varies. Many patients have chronic symptoms and changes to their functional status following their bout with GBS. The Guillain-Barré Syndrome (GBS) Disability Scale is used to measure the degree of recovery. Recovery can range from full recovery for some patients to significant functional impairment for others. The prognosis of GBS depends primarily on age of the patient and on the severity of symptoms two weeks after onset. Older individuals are more likely to have lasting effects following an episode of GBS.
The course of GBS includes progression of symptoms of days to weeks before reaching a plateau period where symptoms stabilize. The plateau period typically lasts weeks but in some cases may last months. Following that there is a gradual recovery where function returns, however, this can take months and many are left with some level of disability.[9]
Roughly 30% of GBS cases will lead to weakness of the respiratory muscles which may eventually lead to respiratory failure[12]. If this occurs, the patient will have to be intubated and placed on mechanical ventilation. Mechanical ventilation can lead to complications such as pneumonia which can affect negatively affect recovery.
Client Characteristics[edit | edit source]
Patient Profile[edit | edit source]
- Initials: D. A (David Atkin)
- Preferred Name: Dave
- Age: 35 years
- DOB: 12 June 1987
- Gender: Male
- Height: 172cm
- Weight: 90kgs
- Significant Presentation: Acute onset of symmetrical bilateral acroparesthesia and paralysis in lower extremities. Patient presented with areflexia and flaccidity bilaterally on testing.
History of Present Illness[edit | edit source]
- Date of Admission: 30th March 2023
- Type of Admission: Self- referral. Patient woke up and was unable to move his lower limbs. Patient brought in an ambulance to Kingston General hospital (KGH).
- Initial Diagnosis: Acute inflammatory demyelinating polyneuropathy (AIDP) a sub-type of Guillain-Barré syndrome. The patient was admitted in KGH on 14th March 2023 with a gastrointestinal infection (caused by Campylobacter Jejuni[1]) which is one of the significant diagnostic indicators.
- Date of Onset: 30th March 2023
- Treatment to Date (20th April 2023): Intravenous immunoglobulin therapy and plasmapheresis, the previous PT and PTA worked on bed mobility.
- Present Status: Alert and oriented. Minimal regain of motor and sensory loss bilaterally on both extremities. (Note: On 15th April 2023, patient was transferred to ICU and intubated after acute bradycardia, bilateral facial paralysis and acute respiratory distress. Patient had shown progressive bilateral symmetrical sensory and motor loss in both upper and lower extremities.)
- Precautions / Contraindications: Patient may exhibit occasional orthostatic hypotension if they are assisted out of recumbent position.
Past Medical History[edit | edit source]
- Allergies: Peanuts and tree nuts
Medication[edit | edit source]
- Venus thromboembolic prophylactics to prevent Deep Vein Thrombosis and Pulmonary Embolism.
- Amlodipine 5mg per day for hypertension.
Health Habits[edit | edit source]
- Smokes one pack every day since he was 30 years old (5 pack-years) and reports drinking 1 beer (3 times a week).
Social History[edit | edit source]
- Patient works as a manager at a software development firm in Kingston.
- The patient lives in a 2-story independent house that has 12 stairs with his wife and 2 daughters (age 9 and 6).
Functional History[edit | edit source]
- Patient did resistance training at GoodLife fitness twice a week.
- Patient was independent with BADLs and IADLs
- Patient fell once while skateboarding and fractured his left wrist in December 2019.
Current Functional Status[edit | edit source]
- Patient has independent bed mobility.
- Patient can maintain seated posture alone but needs assist x1 to transition from laying to sitting and assist x2 to stand.
- Patient unable to ambulate due to balance impairments combined weakness in extremities.
Examination Findings[edit | edit source]
Observation[edit | edit source]
Mobility[edit | edit source]
- Bed mobility: independent to slide up, down, sideways, roll onto side
- Lie to sit: minimal Ax1
- Sit-to-stand: unable to stand on own requires max Ax2
- Transfers: moderate Ax2 required for pivot transfer
Personal Care/ADLs[edit | edit source]
- Assistance needed for dressing and bathing
- Currently utilizing a urinary catheter
- Independent to feed oneself
Gait[edit | edit source]
- Currently unable to ambulate due to lower extremity weakness
- Need fitting for gait aid when required
Range of Motion[edit | edit source]
| Muscle group | AROM | PROM | Muscle Group | AROM | PROM | |
|---|---|---|---|---|---|---|
| All shoulder movements | WNL | WNL | Hip flexion | 45 | WNL | |
| All elbow movements | WNL | WNL | Hip extension | 10 | WNL | |
| All wrist movements | WNL | WNL | Hip adduction | WNL | WNL | |
| Hip abduction | WNL | WNL | ||||
| Knee extension | WNL | WNL | ||||
| Knee flexion | 100 | WNL | ||||
| Dorsiflexion | WNL | WNL | ||||
| Plantar flexion | WNL | WNL |
Muscle Strength[edit | edit source]
| Muscle group | Right | Left | Muscle Group | Right | Left | |
|---|---|---|---|---|---|---|
| All shoulder movements | 4 | 4 | Hip flexion | 3- | 3- | |
| Elbow flexion | 4- | 4- | Hip extension | 3- | 3- | |
| Elbow extension | 4 | 4 | Hip adduction | 4 | 4 | |
| Wrist flexion | 4- | 4- | Hip abduction | 3 | 3 | |
| Wrist extension | 4- | 4- | Knee extension | 3+ | 3+ | |
| Knee flexion | 3- | 3- | ||||
| Dorsiflexion | 3+ | 3+ | ||||
| Plantar flexion | 3+ | 3+ |
Balance[edit | edit source]
- Seated: patient can maintain a seated posture independently once positioned, with SBA
- Standing: unable to independently stand, requires max Ax2
Common Comorbidities[edit | edit source]
- Hypertension
- Diabetes
- Hyperlipidemia
- Acute Respiratory Infection
Outcome Measures[edit | edit source]
- Functional Independence Measure (FIM)
- The FIM is a common outcome measure used to determine the ability to perform activities of daily living. It is made up of 18 individual items consisting of motor and cognitive functioning, that are scored from 1 (total assistance) to 7 (independent). Each item is added together to get an overall level of independence between 18-1126. The FIM is considered time-consuming but an easy-to-use, valid and reliable method that can be trusted in clinical settings to assess patients with various disabilities.
- 10-Meter Walk Test
- The 10-meter walk test is a useful tool to measure gait speed and functional mobility. The tool uses the amount of time a patient takes to walk 10 meters, to provide a speed in m/sec. This can be compared to normative data to determine risk of falls and if a gait aid is indicated. The 10-meter walk test is a common tool used by clinicians and is seen as reliable and valid for a number of neurological conditions.
- Medical Research Council Sumscore
- The Medical Research Council Sumscore is the most common tool used to measure muscle strength in individuals with neurological conditions including GBS. This tool considers six main muscle groups (bilaterally) and assesses their strength using a scale of 0-5. These six scores can then be added together to create a total sum ranging from 0 to 60. It has shown to be a clinically useful tool for physiotherapists.
Clinical Hypothesis[edit | edit source]
Analysis Statement
35 y/o male admitted to Kingston General Hospital (KGH) via ambulance on March 30th, 2023, due to inability to move lower limbs after waking up. Pt presents with bilateral acroparesthesia and paralysis in lower extremities, with bilateral areflexia and flaccidity; diagnosed with acute inflammatory demyelinating polyneuropathy (AIDP) a sub-type of Guillain-Barre syndrome. Pt currently unable to ambulate due to impaired strength, sensory, and balance in lower extremities. This patient's prognosis tends to be positive with a variety of factors that favour his recovery including young age, previously active, and a relatively high MRC Sumscore on initial assessment. However, the patient has several negative factors affecting his prognosis as well, these include having to take care of two daughters, being a current smoker, and inability to ambulate. This patient would benefit from physiotherapy treatment focused on strengthening the lower extremities, improving balance, and increasing mobility.
Problem List[edit | edit source]
- Unable to care for two young daughters (participation in role as father)
- Inability to ambulate (activity)
- Lower extremity weakness in all major muscle groups (body function)
Goals[edit | edit source]
Long-Term Goals
- To be able to pick up and hold daughters safely within the next 4 months
- To be able to walk independently with a 2WW to get to the bathroom within the next 3 months
- Gain the lower extremity strength to be able to perform 10 arm-supported squats within the next 2 months
Short-Term Goals
- To be able to sit up independently to read and interact with daughters within the next week
- Achieve a sit-to-stand and be able to stand with minimal support and a 2WW within the next 3 weeks.
- To be able to complete 10 repetitions of in-bed resistance exercises (glute bridge, quad-over-roll), twice a day within the next 2 weeks
Intervention[edit | edit source]
Treatment Plan
Virtual Motor Rehabilitation System
A key clinical feature of GBS is motor disturbances and muscle weakness that can become persistent and progressive. There has been evidence that traditional motor rehabilitation programs have been beneficial at the onset of this condition to limit the complications of paralysis and with ongoing persistent motor impairments[7]. However, these rehabilitation programs are often tedious and monotonous, leading to decreased adherence.
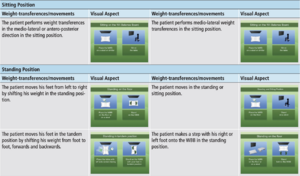
In order to combat the low adherence, a novel virtual motor rehabilitation system has been implemented in two single case studies to assess the effectiveness as an alternative treatment method. An Active Balance Rehabilitation (ABAR) system has been developed for to assist with balance and gait disorders, which utilizes different Virtual Environments to emphasize weight transferences and specific movements that can target symptoms of GBS while addressing other motor impairments.
The characteristics of the ABAR system include:
- A flexible system for the recovery of postural control and for reducing fractures and the risks of falls.
- A suitable system that improves the patient’s motivation and treatment adherence.
- A reinforcement system that allows the results obtained in each session to be monitored and the appropriate action taken.
- A robust system that is able to make a good recovery in parameters such as balance, postural control, muscle tone, and stability in the standing/sitting position in Guillain-Barré patients.
- A portable system that can be used at home to reinforce the acute and sub-acute stages.
- A customizable system that offers multiple levels of difficulty that are based on the patients’ progression.[7]
There are two difficulty levels (low and medium) for the ABAR system, with six different games. This allows for various parameters to be adjusted based on patient specific need including level of difficulty, number of virtual sessions, rest period between virtual sessions, session time, target speed, and target display time. The low difficulty level consists of two virtual environments specific for sitting training, allowing for participants to perform medio-lateral and antero-posterior weight transferences in the sitting position. The medium difficulty level consists of four virtual environments that focus on standing training, based on static or dynamic balance rehabilitation.
The two patients with GBS participating in this novel research design completed three sessions per week for a total of 20 rehabilitation sessions, consisting of 30 minutes of traditional rehabilitation followed by 30 minutes of virtual motor rehabilitation using the ABAR system. The patient’s demonstrated improvements in static and dynamic clinical balance tests following the ABAR intervention, with more significant improvements demonstrated in static clinical balance tests.
Outcome[edit | edit source]
Response to Complications and Adverse Events
| Complication | Risk Factors/When to monitor | Plan |
|---|---|---|
| Pressure ulcers | Prolonged immobility, lack of sensation | |
| Compression neuropathy | Prolonged immobility, lack of sensation | |
| Pain | Impaired communication | |
| Contractures, tissue shortening | Prolonged muscle weakness and immobility |
Referrals
A crucial portion of the health care system is to keep patients’ health care teams broad and interprofessional. The aspect of referring a patient to other health care professionals allows patients to get a well-rounded treatment and care plan, centered around their needs. It has been demonstrated through an overview of studies following GBS patients for 6 months, 1 year and 2 years following their diagnosis, that symptoms can widely range from severity of disability following discharge. (cite Forsberg)
For David, a respiratory therapist would be the first and prioritized referral. About 2 weeks following David’s admission into hospital, he started showing respiratory distress, the preceded to being intubated. Respiratory failure begins to develop during the progressive stage in about a quarter of GBS patients, usually resulting in transfer to the ICU and being mechanically ventilated. (cite Willison). A respiratory therapist would be extremely beneficial for David as they can provide professional advice and treatment to help with current ventilation ability as well as any further prevention of ventilatory complications.
- Monitoring David’s use of accessory muscles, breathing depth, inspiratory and expiratory muscle fatigue/atrophy, coughing ability/strength.
- Drawing blood for monitoring his oxygen levels.
- Education on proper breathing strategies for both the David and his support system
- Proper breathing techniques after extubating.
- Making sure David knows to move arms (within his ability) throughout the day to avoid contractures.
- Providing reassurance to David that around 80% of patients who are mechanically ventilated make extensive recoveries within their first year after onset. (Leonhard)
A second referral for David would be to an Occupational Therapist. David is currently unable to ambulate due to bilateral lower extremity impairment as well as sensory and motor loss to upper extremity as well. An occupational therapist’s role is to assist a patient in the activities of daily living (ADLs), help their home environment become more accessible, fitting and prescribing certain aids that may help people with daily activities (gait aids, reaching objects, sticky mats, etc.).
- OT would assist David with functional exercises that will help him independently carry out ADLs and IADLs.
- With the addition of resistance training if able (cite Ko KJ)
- Assist David with fine motor control of the hand, work will be affected as he uses a mouse throughout the day.
- Help David with sensation in upper extremities, in turn will also contribute to fine motor skills as well.
- Provide education on possible reorganization of household items or rooms to accommodate gait aid as well as making ADLs easier to carry out.
The third referral being made for David would be to a social worker or a psychologist. David has only been diagnosed with GBS for less than a month, this is a severe change in his life, lifestyle, and hobbies. David was previously active and loved to spend time with his wife and two daughters, as well as a successful job in the software development industry. A psychologist would be very beneficial for David to help him with reassurance, connections, and someone to talk to.
- Help connect David with support groups with other patients with GBS and their journeys and experiences.
- Giving David someone to talk to about this traumatic change in his life.
- Help David cope with any stress or anxiety initiated by not being able to care for his wife and daughters like he used to.
- Also, guidance for both David and his wife following this diagnosis, it has been shown that some anxiousness and social dysfunction can occur within family members/relatives (cite Forsberg)
Discussion[edit | edit source]
References[edit | edit source]
- ↑ 1.0 1.1 1.2 Willison HJ, Jacobs BC, van Doorn PA. Guillain-barre syndrome. The Lancet. 2016 Aug 13;388(10045):717-27.
- ↑ 2.0 2.1 2.2 Hughes RA, Cornblath DR. Guillain-barre syndrome. The Lancet. 2005 Nov 5;366(9497):1653-66.
- ↑ Abara WE, Gee J, Marquez P, Woo J, Myers TR, DeSantis A, Baumblatt JA, Woo EJ, Thompson D, Nair N, Su JR. Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States. JAMA Network Open. 2023 Feb 1;6(2):e2253845-.
- ↑ Ropper AH. The Guillain–Barré syndrome. New England journal of medicine. 1992 Apr 23;326(17):1130-6.
- ↑ Van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, Van Doorn PA. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nature Reviews Neurology. 2014 Aug;10(8):469-82.
- ↑ Alshekhlee A, Hussain Z, Sultan B, Katirji B. Guillain–Barré syndrome: incidence and mortality rates in US hospitals. Neurology. 2008 Apr 29;70(18):1608-13.
- ↑ Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. The lancet. 2021 Mar 27;397(10280):1214-28.
- ↑ Walling A, Dickson G. Guillain-Barré syndrome. American family physician. 2013 Feb 1;87(3):191-7.
- ↑ 9.0 9.1 Wijdicks EF, Klein CJ. Guillain-barre syndrome. InMayo clinic proceedings 2017 Mar 1 (Vol. 92, No. 3, pp. 467-479). Elsevier.
- ↑ 10.0 10.1 Govoni V, Granieri E. Epidemiology of the Guillain-Barré syndrome. Current opinion in neurology. 2001 Oct 1;14(5):605-13.
- ↑ 1. GBS (Guillain-Barré Syndrome) and vaccines [Internet]. Centers for Disease Control and Prevention; 2023 [cited 2023 May 10]. Available from: https://www.cdc.gov/vaccinesafety/concerns/guillain-barre-syndrome.html
- ↑ Hahn AF. The challenge of respiratory dysfunction in Guillain-Barré syndrome. Archives of Neurology. 2001 Jun 1;58(6):871-2.