Burn Wound Treatment: Cleansing and Solutions
Top Contributors - Stacy Schiurring and Jess Bell
Introduction[edit | edit source]
Providing care to patients with burn wound injuries is a complex undertaking and requires collaborative management from an interdisciplinary team. The wound care professional providing care at the bedside must take the overall medical condition of the patient into consideration: (1) immunosuppression, (2) the extent of the burn wound, and (3) medical comorbidities,[1]
"Burn wound cleansing is an integral step in every wound management protocol. Yet a lot of this practice is based on myth rather than real scientific basis." [2]
Infection prevention, oedema management, and effective skilled wound care all contribute to proper healing of a burn wound injury. Wound cleansing is an important part of wound bed preparation.[3] Unfortunately, there is not much evidence that supports a particular type of wound cleansing for burn wounds.[4] This article will explore the available literature and provide the best available evidence for burn wound management and cleansing recommendations.
Burn Wound Cleansing[edit | edit source]
Wound cleansing is the removal of surface contaminants, loose debris, slough, softened necrosis, microbes and/or remnants of previous dressings from the wound surface and from the periwound skin.[3]
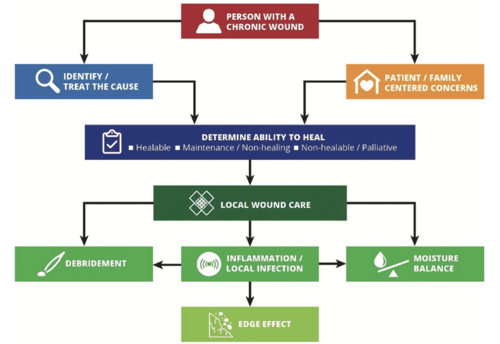
Burn wounds are much more fragile when compared to other wound types. Therefore a more gentle mechanical force is required when cleaning a burn wound, newly formed epithelium and the new onset of growing epithelium from the wound margins can easily be damaged.[4]
Infection in a burn wound can also negatively affect the growing epithelium and granulation tissue and stop the healing progress. When a burn wound is visibly infected or infection is confirmed by laboratory findings it is appropriate to use a slightly more aggressive approach to wound cleansing and topical agents. However, once the infection is under control, return to more gentle cleansing and less cytotoxic topical agents which encourage the burn wound to progress to healing.[4]
Client pain management is vital for effective and thorough wound cleansing.[4] Fear of pain during wound care is often a top concern for clients.
Every client has the right to proper pain management, this can be remembered using the "six-C's." Every client deserves:[5]
- to be Checked for pain before during and after the treatment
- the have the Cause of their pain determined
- the have the Consequences of treatment explained
- to have adequate pain Control
- to have the ability to Call time-outs during procedures
- to have Comfort
It is important to properly document pain management and pain ratings in the medical record.[5] To learn more in-depth information about pain mechanisms, please read this article. To read more about general pain assessment techniques, please read this article.
Goals of Wound Cleansing[4][edit | edit source]
- To minimise the risk of wound infection by removing potential sources of contamination from the wound surface.
- To remove any residual topical agents and drainage that has been collecting on the wound surface since the last dressing change because they can create an environment for microbial growth.
- To remove any debris or loose, non-viable tissue from the surface of that wound to decrease infection risk. The presence of non-viable tissue can also slow down the healing process.
- To provide a clean environment for more reliable would culture sample or biopsy collection.
- To hydrate the surface of the wound which can help preserve tissue viability and encourage further wound healing.
Wound Cleansing Considerations[edit | edit source]
- Sterile versus non-sterile solution. There is not strong literature support on the use of sterile versus non-sterile solution to clean the wound. Rather, the decision should be based on the wound care professional's wound assessment and clinical judgement.
- Volume. An adequate amount of solution must be used to effectively clean a burn wound, however there is not a defined volume per literature review. Enough solution should be used to thoroughly clean the wound surface.
- Force. When cleansing a burn wound with mechanical force, either manually or with a device, ensure that force is appropriate for that wound using clinical judgement. The literature does not define the amount of force needed to properly clean a burn wound.
- Temperature. Solutions should be warmed to room temperature or slightly warmer per literature review. Typically temperatures range from low to mid 90oF (32-35oC).
- Aseptic Technique. Use aseptic (clean) technique with appropriate personal protective equipment (PPE).
- Periwound. It is important to properly cleanse the periwound area because any skin under the dressing has the potential to spread unwanted bacteria into the burn wound. Proper cleansing and moisture barrier protection will also prevent periwound maceration and irritation.
ADD VIDEO ON CLEAN TECHNIQUE?
Wound Cleansing Strategies: Hydrotherapy[edit | edit source]
Water can be used in many different ways to treat medical conditions. According to the Centers for Disease Control and Prevention (CDC), "hydrotherapy involves the use of water for soothing pains and treating certain medical conditions" using equipment ranging in size from swimming pools to whirlpool tanks.[6]
Hydrotherapy has a been a part of burn wound care since the 17th century. It can be performed by various methods, such as: immersion, shower, bedside irrigation or wiping. Despite its long history of use, there continues to be controversy around the most optimal method for cleansing burn wound injuries, and further research and clinical studies are needed. Due to the lack of evidence-based recommendations, most burn treatment centres are guided by wound care professional's clinical experience.[7]. According to a study published in 2022, there are no current recommendations for the daily use of hydrotherapy for burn wound treatment. The study also points out that some research authors do not consider hydrotherapy as part of normal routine burn care mainly due to the risk of infection transmission when proper cleaning and preparation precautions are not observed.[7]
Whirlpool Tank Immersion[edit | edit source]
Whirlpool therapy (WP therapy) can be used to treat burn wounds in need of extensive debridement. This cleansing strategy can utilise water jet agitation and the addition of antimicrobial agents to further aide in cleansing and mechanical debridement. A full-body tank, such as a Hubbard tank, or a smaller extremity tank can be selected based on the location of the wound.[8]
ADD IMAGES OF WP TANKS
Benefits:
- effectively cleans the surface of the burn wound by decreasing gross contaminants and toxic debris including surface bacteria. This process can be increased with use of the whirlpool turbine to agitate the water to provide mechanical debridement.[4][8]
- thoroughly hydrates the burn wound[4]
- increase local circulation[8]
- decrease wound pain[8]
- decrease fever[8]
- help soak and gently remove dressings[8]
- ultimately accelerate healing[8]
Risks:
- physiologically taxing on the patient[4]
- can develop hypothermia very quickly after removal from warm water[4]
- there is significant risk of infection, especially with full body immersion[4]
- risk of infection from equipment. Biofilms can form on whirlpool equipment or in water pipes and allow bacteria to evade antiseptics, antimicrobials, and even the most rigorous sterilization procedures.[4][8]
VIDEO ON BIOFILM ON SURFACES?
Showering[edit | edit source]
This method involves exposing the burn wound to running water in a shower setup. A slightly cooler water temperature should be used during this method of burn wound cleansing. It is recommended that the room temperature be warm to protect the client from getting too cold due to hypothermia risk.[4]
Benefits:[4]
- less physiologically stressful than immersion method
- minimises the risk of cross-contamination
Risks:[4]
- hypothermia risk
- aggressive force of water hitting wound bed from shower head. To modify the force, allow the water spray to hit the intact skin and run over the surface of the wound to flush that wound of contaminants.
- risk of infection from equipment due to presence of biofilms, however the risk is less than the immersion method.
Modifications and Considerations:
- In situations where there is limited or no access to a shower, the cleansing solution can be poured over the burn wound from a properly disinfected glass or metal vessel.[4]
- The immersion method can be followed up with a shower to remove any potential contaminates.[4][7]
- When selecting bathroom equipment, select simple equipment which is easy to disinfect. Use single use disposable sheets and drapes.[7]
Soaking and Wiping[edit | edit source]
This technique is common in clinical practice. Soaking is performed by saturating sterile gauze or cloth with a cleansing solution, then covering the entire surface of the burn wound.[7] There is not a determined amount of time for soaking, rather it is based on the wound care professional's wound assessment findings and clinical judgement. The wound surface is then wiped to perform mechanical debridement. Research has found that the mechanical force needed to remove surface debris from a non-burn wound without causing tissue damage ranges from 4 to 15 pounds per square inch (psi). There is no defined pressure for a burn injury.[4]
This method is very effective for small burn wounds, superficial partial thickness burn wounds, or well healing burn wound injuries that require only light pressure for cleansing to protect new healing epithelium.[4]
Benefits:[4]
- soaking provides hydration to the wound
- use of antimicrobial agent decreases risk of cross-contamination
- mechanical debridement of wiping removes contamination, slough, and debris from wound surface
Risks:[4]
- difficult to main consistent force when wiping the wound's surface of that wound
Wound Cleansing Solutions and Additives[edit | edit source]
Cleansing Solutions[edit | edit source]
Common cleansing solutions include:
- sterile saline
- sterile water
- distilled water
- potable water
- commercially available wound cleansers
These listed cleansers are considered to be non-antimicrobial, inert, and non-cytotoxic cleansing solutions. They all require some mechanical force to be effective in cleaning the burn wound surface.
Make this into a special topic box? One of the things about potable water, if you're using tap water or well water, depending on where you are and what the source of that water is, it can be very contaminated. So you want to be careful when you're using potable water that you aren't using something that could have come from a contaminated source, or you would want to boil it first, and then let it cool before you used it on that patient.
Topical antimicrobial agents[edit | edit source]
Antimicrobials are a group of agents and substances which reduce the possibility of infection and sepsis[9]:
- Antibiotics are naturally derived from other microorganisms or synthetically created agents which are absorbed into the body for the purpose of killing or preventing bacterial multiplication. These substances can be given parenterally (intramuscularly, intravenously), orally, or applied topically to the skin.[9]
- Antiseptics are chemical agents that slow or halt the growth of microorganisms on external body surfaces, including wounds, and aid in preventing infection.[10] These substances are non-damaging to living tissue and skin.[11]. When applied to the skin antiseptics are not absorbed in a significant quantity into the body.[9]
- Disinfectants destroy microorganisms on non-living objects or surfaces.[9]
- Antifungals agents are agents that slow or halt the growth of fungi, including yeasts. These substances can be given intravenously, orally or topically.[9]
The purpose of the topical antimicrobial agents is to decrease the bioburden on the surface of the burn wound. They are used for their biocidal effect on bacteria, fungi, parasites, and viruses.
Indications for use[edit | edit source]
Guidelines for when to use a topical antimicrobial or antiseptic agent:
- patients with a high risk of infection
- patients that are showing clinical signs of a local infection (this can be used in combination with a systemic antibiotic in the event of a spreading or systemic infection)
- wounds with an assumed or known biofilm formation (can also be used in combination with surgical debridement and systemic antibiotics)
Guidelines for selecting a topical antimicrobial agent:
- it should be broad spectrum
- it should have a known efficacy of treatment against suspected or known infection sources in the burn wound
- it should have no or low cytotoxicity to the healthy wound bed
- it should be not irritating the patient
- the patient should have no known allergy to any of the agent's components
- it should be fast acting and long lasting
- no or limited known bacterial resistance to the agent
- it needs to be locally available
- has guidelines for use in clinical practice
The burn wound should be reassessed with each dressing removal and change, and the treatment plan of care adjusted as appropriate.
Special Topic: Cytotoxicity refers to the ability of a substance or process to damage cells and cause cell death, including the death of healthy cells and viable tissue in a wound bed.
When treating a significant infection, it can be more important to kill the invading bacteria at the risk of some healthy cell loss before the infection can become established. Infection in a wound bed will disrupt the expected wound healing process, destroy healthy viable tissue in the wound bed, and cause the wound to deteriorate. The wound care professional must be able to find a balance between initial viable tissue damage due to the cytotoxicity of the topical antimicrobial agent and allowing the infection to take hold and also cause damage and delay wound healing and closure.
List of Topical Agents[edit | edit source]
Below is a non-exhaustive list of common of topical agents.
Burn Wound Cleansing Solutions
| Solution Name | Description | Indications/Uses | Contraindications/Risks |
|---|---|---|---|
| Mafenide acetate (Sulfamyalon) | 5% solution |
|
Use creates a risk for the patient developing metabolic acidosis, which is particularly a risk for patients who are in respiratory distress. |
| Povidone iodine (Betadine) |
|
|
|
| Acetic acid |
|
|
|
| Hypochlorous acid | a super-oxidised solution (meaning it contains hypochlorous acid and sodium hypochlorite. These solutions are naturally acidic due to increased hydrogen ions concentration)[12] |
|
|
| Sodium hypochlorite (Dakin's Solution) |
|
0,025% sodium hypochlorite: Heggers found that with contact up to 30 minutes in a, of course it was an in vitro study, the effectiveness of this concentration against bacteria was extremely successful through a broad range of bacteria, but it was not cytotoxic. They did find that the fibroblasts and the keratinocytes sloughed from the surface of the wound, but they remained viable in the solution that surrounded that wound. So they felt like even though they had slough from the surface, they were still active and effective in helping against the microbial growth and to help that wound heal. So 0,025% was our standard of care, not just with burn wounds, but with non-burn wounds as well in trying to maintain an appropriate and manageable level of bioburden on the surface of the wound. | Dakin's was found to be, in its original formula, extremely cytotoxic, although it did also eradicate any microbial infection that was happening on the wound bed. |
Other cleansers
| Solution Name | Description | Indications/Uses | Contraindications/Risks |
|---|---|---|---|
| Polyhexamethylene biguinide (PHMB) | being used as an additive to dressings, gauze dressings and foam dressings and not really as a cleansing agent | I mentioned it because they're potentially, they could come out with some kind of a solution that contains it because it is broad spectrum against Gram-negative and Gram-positive microbes as well as fungi, viruses, MRSA, (methicillin-resistant Staphylococcus aureus) and biofilms. | |
Other solutions are also commercially available that are designed to help with burn wound cleansings. Many of them contain a surfactant. A surfactant has the additional benefit of separating the layer of non-viable tissue. Not necessarily eschar, but that pseudo-eschar and that slough and some of that coagulated debris that sits on the surface of the wound. It helps separate it from the underlying viable wound bed by breaking those bonds that are holding that material to the wound bed. And that not only helps with the mechanical debridement and cleaning of that burn, but it allows the antimicrobial agent to get to the surface of the burn wound and provide that antimicrobial coverage more effectively.
Chlorhexidine and soap are also mentioned and known to be used in clinical practice. One of their benefits is they have the surfactant, which allows for a good mechanical cleaning of the surface of the wound. However, there's no dosage control with either one of those. Chlorhexidine in its formulated strength is extremely cytotoxic and it needs to be diluted with water, but there's no formula for how much to dilute it, and it's very inconsistent in how that's done in clinical practice as well as soaps. So potentially it can remain cytotoxic. It's very important if you're using that to clean the burn wounds, particularly in the surgical setting, that you rinse it from the burn wound thoroughly so that there's no cytotoxic residual effect from those products.
And then there are commercial wound cleansers available. Most of them also have a surfactant added to them, and most of them are also antimicrobial. When they first came onto the market, they were very cytotoxic. Almost all of them were too cytotoxic for use, so they were all reformulated, and they now, to my knowledge, they're all less potent and therefore not cytotoxic to the wound bed and can be used very effectively, especially for smaller burn wounds or in the home setting.
Hydrogen peroxide is another agent that has long been used for cleaning wounds, not necessarily burn wounds, but wounds in general. But its standard concentration, which is 3%, is extremely cytotoxic to fibroblasts, keratinocytes, macrophages, and can stop wound healing or actually cause it to deteriorate if used for too long. When I first started doing wound care in the mid-80s, hydrogen peroxide was very normally given to a patient with instructions to use it every day until their wound healed and many wounds took a long time to heal because that hydrogen peroxide was so toxic. So it's not common to use it for burn wound injuries, but one of the benefits of hydrogen peroxide is the effervescing action that it has when it touches the wound bed and it becomes oxygenated and that effervescence can help loosen debris, dried blood, contaminants that are sort of adherent to that wound bed so that you can then mechanically wipe them away or debride them away. So it's effective if you use it for one or two treatments for a specific purpose in a defined area, but then you also want to make sure you rinse that product away before you bandage the burn wound so there's, again, not that lingering effect that can be cytotoxic to the tissue.
Special Topic: planktonic bacteria versus biofilms?
Wound Cleansing Frequency[edit | edit source]
Once you have determined the type of wound cleansing intervention you're doing and the topical agent that you're using to take care of that burn wound, the next part of the plan is to decide how often that intervention needs to happen. So all of these topical agents that I talked about today are not sustained-released or time-released antimicrobial products. They have a very short-term effectiveness in being antimicrobial. So if you're just using them for cleansing and you're using a dressing that is antimicrobial with a time-dependent feature, you know, change it every three days or every five days, then the frequency of intervention is based on that particular dressing and the recommendations for the frequency of dressing changes. However, many of these solutions are also used to saturate the dressings that are applied. Just plain gauze that is not antimicrobial other than this topical agent that we're adding, or any other inert dressing that you're saturating with this particular topical agent. Those do not have that sustained release and need to be changed a minimum of once a day and that daily also pertains to wounds that have just a low amount of drainage, no real signs of infection, and they just need to be kept clean.
The dressing change needs to be done, at least BID (twice a day) with increasing drainage and increasing signs of infection, and potentially those patients that are at really high risk for developing an infection if care isn't done very strategically. They need to be changed even more often than BID when the signs of infection are severe, you have copious amounts of drainage, the wound is deteriorating, the patient has active signs of clinical infection. Then that care needs to be every eight hours, every six hours, even potentially every four hours to get that microbial bioburden off the surface of the wound. And you also want to use more vigorous techniques like I talked about earlier, to eradicate that bioburden from the surface of the wound. So you're putting at risk some of the healthy tissue to remove that infection that will destroy that healthy tissue if you don't get rid of it, as I said before.
And the frequency of dressing changes may vary in different parts of the body on the same patient. If they have an arm burn and a leg burn and a trunk burn, and they're all presenting at different levels of symptoms of infection or the progress toward healing, then the frequency of each of those areas may be different. You may be changing the arm burn every six hours and the leg burn every two or three days because you have an antimicrobial dressing on it. So really the assessment piece, as you're changing that dressing, will dictate how you're treating that burn, how often, what agents that you're using, and you may go back and forth from an antimicrobial agent to an inert agent as the picture of that burn wound changes with each dressing change.
So these pictures are designed just to kind of summarise what we've talked about with our topical agents today. If a patient presents with a very high risk of infection or a high risk of infection and they're having copious exudate, then you really need to be aggressive in how you're cleaning that burn wound and the topical antimicrobial agent that you're using. And it could be combined with surgical debridement as well, because once they're surgically debrided, you really need to clean that wound surface so any remaining bacteria can be eradicated as best as possible. So deep like full-thickness or deep partial-thickness burn wounds, large surface area burns, burns that have had eschar that hasn't been debrided and has been present for quite a while, burns that are already presenting with the look of infection. All of those really need aggressive frequent intervention to minimise that risk of infection and eradicate the microbial bioburden. When the burn is superficial partial-thickness, it's very small, it's showing signs of healing with epithelial budding throughout the burn wound, as well as new epithelial growth from the edges, then you want to be very gentle with that burn care, using inert products, not antimicrobial products, using very gentle care so that epithelial growth can continue and won't be destroyed by the interventions that we provide.
Conclusion[edit | edit source]
So in conclusion, when you're trying to decide what type of wound cleansing you're going to provide the burn patient and what topical agents you may use in cleaning the burn wounds, remember that unfortunately, there's not a lot of supportive evidence, but you do want to find evidence that will at least help you initiate that first intervention and the thought process behind it, and then you're assessing that burn wound each time you do the dressing change to make sure you're either getting the results that you want or if you're not, that you're revising your intervention to try to achieve those results.
There is quite a bit of expert agreement in using antimicrobial agents, especially when you're looking at the development of a local infection or even a more serious infection. And to be used in combination with systemic antibiotics for those more serious infections, as well as for management of biofilms and those patients that are at very high risk for developing an infection.
As I said, once the signs of infection have resolved, the wound shows really good healing, which means there isn't an infection or the wound wouldn't continue to heal, you may discontinue the use of antimicrobial agents and use the more inert agents. Again, remembering you may need to switch back and forth until that burn wound has effectively re-epithelialised.
Resources[edit | edit source]
- Pain measurement tools
- x
or
- numbered list
- x
References[edit | edit source]
- ↑ Markiewicz-Gospodarek A, Kozioł M, Tobiasz M, Baj J, Radzikowska-Büchner E, Przekora A. Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. International Journal of Environmental Research and Public Health. 2022 Jan 25;19(3):1338.
- ↑ Hayek S, El Khatib A, Atiyeh B. Burn wound cleansing-a myth or a scientific practice. Annals of burns and fire disasters. 2010 Mar 3;23(1):19.
- ↑ 3.0 3.1 Rodeheaver GT, Ratliff CR. Wound cleansing, wound irrigation, wound disinfection. InChronic wound care: A clinical source book for healthcare professionals 1997 (pp. 97-108). Health Management Publications, Wayne, Pa.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 Merwarth, D. Management of Burn Wounds Programme. Burn Wound Cleansing and Solutions. Physioplus. 2023.
- ↑ 5.0 5.1 Sibbald RG, Elliott JA, Persaud-Jaimangal R, Goodman L, Armstrong DG, Harley C, Coelho S, Xi N, Evans R, Mayer DO, Zhao X. Wound bed preparation. World Council of Enterostomal Therapists Journal. 2022 Mar 1;42(1):16-28.
- ↑ Centers for Disease Control and Prevention. Water Use in Hydrotherapy Tanks. Available from: https://www.cdc.gov/healthywater/other/medical/hydrotherapy.html (accessed 21 March 2023).
- ↑ 7.0 7.1 7.2 7.3 7.4 Tiglis M, Peride I, Neagu TP, Raducu L, Lascar I. Hydrotherapy in burn care: Pros, cons and suggestions. Romanian medical JouRnal. 2022 Jan 1;69(1):15.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Tao H, Butler JP, Luttrell T. The role of whirlpool in wound care. Journal of the American College of Clinical Wound Specialists. 2012 Mar 1;4(1):7-12.
- ↑ 9.0 9.1 9.2 9.3 9.4 Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Antimicrobials including antibiotics, antiseptics and antifungal agents. InScoping systematic review of treatments for eczema 2016 May. NIHR Journals Library.
- ↑ DermNet. Antiseptics. Available from: https://dermnetnz.org/topics/antiseptic (accessed 26 March 2023).
- ↑ Suzuki JB, Misch CE. Periodontal and maintenance complications. InMisch's Avoiding Complications in Oral Implantology 2018 Jan 1 (pp. 771-826). Mosby.
- ↑ Haesler E. Evidence Summary: Super-oxidised solutions for chronic wounds. Wound Practice & Research: Journal of the Australian Wound Management Association. 2020 Sep 1;28(3):145-7.






