Hierarchy of evidence: Difference between revisions
Kim Jackson (talk | contribs) No edit summary |
Kim Jackson (talk | contribs) No edit summary |
||
| Line 12: | Line 12: | ||
[[File:Picture4.png|right|400x400px]] | [[File:Picture4.png|right|400x400px]] | ||
== Systematic Review | == Systematic Review== | ||
A systematic review is a form of research that provides a summary of medical reports on a specific clinical question, using explicit methods to search, critically appraise, and synthesise the world literature systematically. It is particularly useful in bringing together a number of separately conducted studies, sometimes with conflicting findings, and synthesising their results. By providing in a clear explicit fashion a summary of all the studies addressing a specific clinical question, systematic reviews allow us to take account of the whole range of relevant findings from research on a particular topic, and not just the results of one or two studies.They can be used to establish whether scientific findings are reliable and generalised across populations, settings, and treatment variations, or whether findings vary significantly by particular subgroups. | |||
{{#ev:youtube|lfO5yB9EoJU}} | {{#ev:youtube|lfO5yB9EoJU}} | ||
{{#ev:youtube|Ky4bU94dZnY}} | {{#ev:youtube|Ky4bU94dZnY}} | ||
{{#ev:youtube|MEBal2QgH7c}} | {{#ev:youtube|MEBal2QgH7c}} | ||
== Meta-Analysis | == Meta-Analysis == | ||
Following a systematic review, data from individual studies may be united quantitatively and reanalysed using established statistical methods. This technique is called a meta-analysis. The justification for a meta-analysis is that, by combining the samples of the individual studies, the overall sample size is increased, thereby improving the statistical power of the analysis as well as the precision of the estimates of treatment effects. | Following a systematic review, data from individual studies may be united quantitatively and reanalysed using established statistical methods. This technique is called a meta-analysis. The justification for a meta-analysis is that, by combining the samples of the individual studies, the overall sample size is increased, thereby improving the statistical power of the analysis as well as the precision of the estimates of treatment effects. | ||
== Randomized Controlled Study | == Randomized Controlled Study== | ||
An experimental design used for testing the effectiveness of a new medication or a new therapeutic procedure. <ref name="htm" />Individuals are assigned randomly to a treatment group (experimental therapy) and a control group (placebo or standard therapy) and the outcomes are compared. | An experimental design used for testing the effectiveness of a new medication or a new therapeutic procedure. <ref name="htm" />Individuals are assigned randomly to a treatment group (experimental therapy) and a control group (placebo or standard therapy) and the outcomes are compared. | ||
| Line 34: | Line 34: | ||
[[Image:Picture2.png]] | [[Image:Picture2.png]] | ||
== Case-Control Study | == Case-Control Study == | ||
A non-experimental research design using an epidemiologic approach in which previous cases of the condition are used in lieu of new information gathered from a randomized population. | |||
A group of patients with a particular disease or disorder, such as myocardial infarction, is compared with a control group of persons who have not had that medical problem. | A group of patients with a particular disease or disorder, such as myocardial infarction, is compared with a control group of persons who have not had that medical problem. | ||
| Line 44: | Line 44: | ||
[[Image:Picture3.png]] | [[Image:Picture3.png]] | ||
== Case Report | == Case Report== | ||
In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient but usually describe an unusual or novel occurrence. | In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient but usually describe an unusual or novel occurrence. | ||
| Line 52: | Line 52: | ||
an article in a newspaper or other publication presenting the opinion of the publisher, editor, or editors. <br> | an article in a newspaper or other publication presenting the opinion of the publisher, editor, or editors. <br> | ||
== Reference | == Reference== | ||
<references /> | <references /> | ||
[[Category:EBP]] | [[Category:EBP]] | ||
Revision as of 05:20, 19 October 2022
Original Editors -Andeela Hafeez
Top Contributors - Andeela Hafeez, Kim Jackson, Shaimaa Eldib, WikiSysop, 127.0.0.1 and Candace Goh
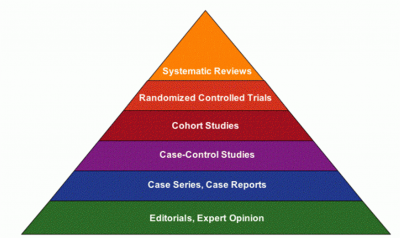
Hierarchy Of Evidence[edit | edit source]
Hierarchy or evidence sometimes referred to as level of evidenceis the core principle of evidence based practice. It explains how to find the best evidence from the top down, by first looking for a recent well-conducted systematic review and then moving down to the next level of evidence to support your statement or answer your question.[1]
Systematic Review[edit | edit source]
A systematic review is a form of research that provides a summary of medical reports on a specific clinical question, using explicit methods to search, critically appraise, and synthesise the world literature systematically. It is particularly useful in bringing together a number of separately conducted studies, sometimes with conflicting findings, and synthesising their results. By providing in a clear explicit fashion a summary of all the studies addressing a specific clinical question, systematic reviews allow us to take account of the whole range of relevant findings from research on a particular topic, and not just the results of one or two studies.They can be used to establish whether scientific findings are reliable and generalised across populations, settings, and treatment variations, or whether findings vary significantly by particular subgroups.
Meta-Analysis[edit | edit source]
Following a systematic review, data from individual studies may be united quantitatively and reanalysed using established statistical methods. This technique is called a meta-analysis. The justification for a meta-analysis is that, by combining the samples of the individual studies, the overall sample size is increased, thereby improving the statistical power of the analysis as well as the precision of the estimates of treatment effects.
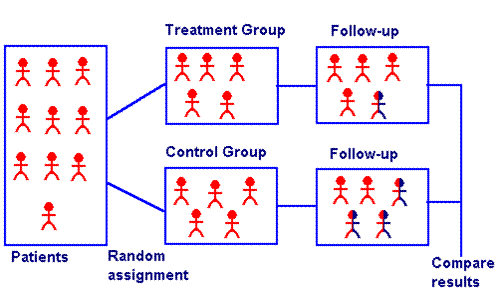
Randomized Controlled Study[edit | edit source]
An experimental design used for testing the effectiveness of a new medication or a new therapeutic procedure. [2]Individuals are assigned randomly to a treatment group (experimental therapy) and a control group (placebo or standard therapy) and the outcomes are compared.
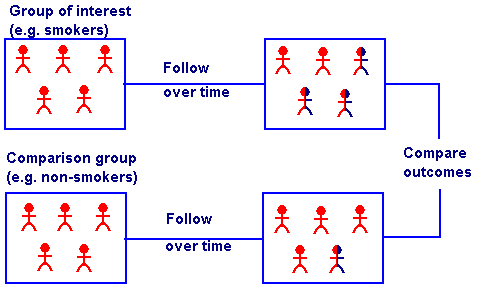
Cohort Study[edit | edit source]
Analytical study in which a group having one or more similar characteristics (such as habit of smoking or a particular disease) is closely monitored over time simultaneously with another group (whose member do not smoke or are free from the disease).[3]
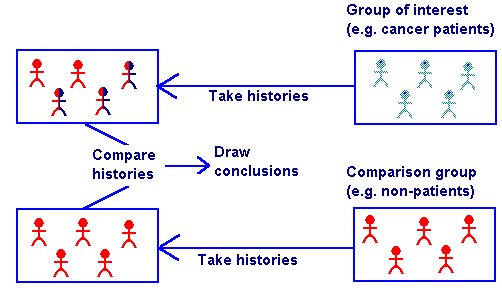
Case-Control Study[edit | edit source]
A non-experimental research design using an epidemiologic approach in which previous cases of the condition are used in lieu of new information gathered from a randomized population.
A group of patients with a particular disease or disorder, such as myocardial infarction, is compared with a control group of persons who have not had that medical problem.
The two groups, matched for age, sex, and other personal data, are examined to determine which possible factor (e.g., cigarette smoking, coffee drinking) may account for the increased disease incidence in the case group.[2]
Case Report[edit | edit source]
In medicine, a case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient but usually describe an unusual or novel occurrence.
Editorial:[edit | edit source]
an article in a newspaper or other publication presenting the opinion of the publisher, editor, or editors.